The European Respiratory & Anesthesia Devices Market, mainly driven by geriatric population and increasing prevalence of Chronic Obstructive Pulmonary Diseases (COPDs) and Obstructive Sleep Apnea (OSA) accompanied by rapid rise in the number of surgical procedures was estimated at $3.2 billion and is expected to reach $5.0 billion by 2018, at a CAGR of 9.2% from 2013-2018. The Respiratory devices market constitutes the largest segment of the European Respiratory and Anesthesia Devices Market.
The report “European Respiratory & Anesthesia Devices Market forecast, 2012-2018” analyzes the market of devices by 2 segments such as Respiratory & Anesthesia devices. All of these segments experience a substantial positive growth owing to the utmost acceptance of home healthcare devices. The Positive airway pressure devices segment has been contributing majorly towards total Respiratory Devices industry in Europe. European Respiratory and Anesthesia devices market contributed around 53% to the global Respiratory and Anesthesia devices market in 2013. Both the markets have been witnessing the maximum growth because of increase in patient pool and rise in incidence of respiratory diseases like Obstructive Pulmonary Diseases (COPDs) and Obstructive Sleep Apnea (OSA).
Germany has been accounting majorly for the European Respiratory and Anesthesia Devices Market owing to increasing healthcare awareness, availability of reimbursement, improving healthcare infrastructure, and economic growth favored by broadening insurance coverage and increased number of outpatient surgeries.
Growing healthcare cost containment pressure in the Euro region has caused a downtrend in the growth of Respiratory and Anesthesia Devices market. Tax reforms backed by increased regulatory compliance norms are also restraining the progress and profitability of the European Respiratory and Anesthesia Devices market.
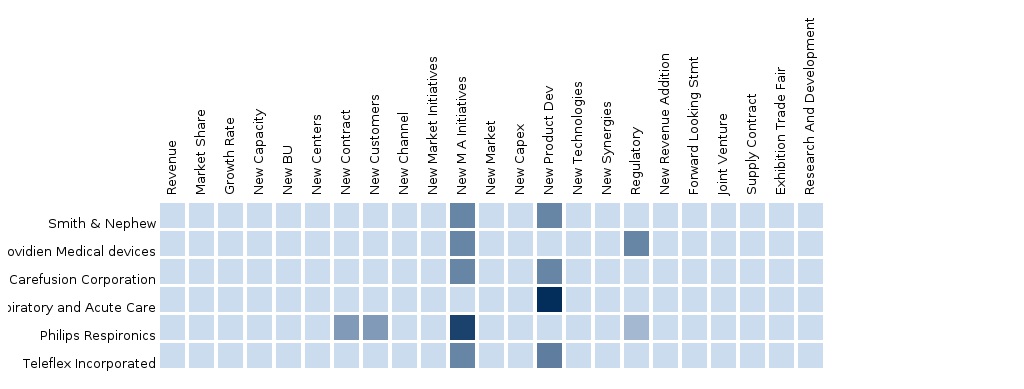
The report also provides a detailed competitive landscaping of Companies operating in this market. Segment and Country specific Company shares, News & Deals, M&A, segment specific pipeline products, product approvals and product recalls of the major companies would be detailed. The main companies operating in this market in Europe are Covidien plc, Smiths Medical, GE Healthcare, Teleflex Incorporated, Draegerwerk AG & Co. KGaA, Intersurgical Ltd. and Vincent Medical Mfg. Co., Ltd.
Customization Options:
Along with the market data, you can also customize MMM assessments that meet your company’s specific needs. Customize to get comprehensive industry standard and deep dive analysis of the following parameters:
Product Analysis
- Usage pattern (in-depth trend analysis) of products (Segment wise)
- Product Matrix which gives a detailed comparison of product portfolio of each company mapped at country and sub segment level
- End-user Adoption rate analysis of the products (Segment wise and Country wise)
- Comprehensive coverage of Product approvals, Pipeline products and Product recalls and Refurbished market
Epidemiology Data
- Country specific Prevalence of Respiratory diseases like Chronic Obstructive Pulmonary Diseases (COPDs) and Obstructive Sleep Apnea (OSA)
- Country specific Patient pool of Chronic Obstructive Pulmonary Diseases (COPDs) and Obstructive Sleep Apnea (OSA)
- Disease progression (Pattern analysis)
Ancillary Data
- Number of unit sales along with the respective ASPs annually in each country tracked till sub-segment level in the Respiratory and Anesthesia devices market
- Usage data and purchasing data in this market
- Number of hospitals, diagnostic clinics.
Surgeons/Physicians Perception analysis
Fast turn-around analysis of Surgeons response to Respiratory & Anesthesia devices market events and trends
- Pattern analysis of usage of anesthesia devices by physicians and anesthesiologists
- Surgeons opinion about products from different companies
- Surgeons qualitative inputs on epidemiology data
Brand/Product Perception Matrix
Comprehensive study of customers Brand/Product Perception Matrix and behavior through our inbuilt social connect tool (digital marketing language) checking the virality and tonality of blogs
- Analysis of overall brand usage and familiarity and brand advocacy distribution (Detractor/Neutral/Familiar) in this market
Competitive Intelligence
- Company share analysis of the key players of this market forecasted till 2018 across the sub-segments, country-wise.
- Develop business strategies to enhance the bottom line of the companies
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
Brazil Medical Devices Peers of Brazil medical devices are Orthopedic Devices, Ophthalmology devices, Endoscopy and Neurology Devices comprising 12.8%, 9.2%, 7.2% and 1.9% respectively of the Global Medical Devices market. It is segmented on basis of endusers and... |
Nov 2015 |












