The European plastic surgery biomaterials market has seen challenging and dynamic market conditions in recent years. The European market is mainly driven by increased investments, funds and grants by government bodies worldwide, precipitous growth anticipated for plastic surgery and wound healing applications, growing number of elderly people, technological advancements, and changing lifestyles.
The report analyzes the market in terms of technology devices, such as craniofacial surgery, soft tissue fillers, acellular dermal matrices, peripheral nerve repair, facial wrinkle treatment (hyaluronic acid-based), and bio engineered skins.
Incessant rise in the number of collaborations, conferences, and research-related activities are also propelling the growth of the plastic surgery biomaterials market globally. Immunological and inflammatory reactions, stringent regulatory systems, issue of fatigue, wear, and reimbursement concerns are the major restraints of the plastic surgery biomaterials market. The European plastic surgery biomaterials market witnesses a plethora of growth opportunities. Biomaterials find wide application in the field of wound healing market as a result of the novel developments of biomaterials for plastic surgery, colossal pool of patients in the region, rise of plastic surgery biomaterials market in countries such as Germany, and increased conferences and research-related activities in the region. The burning issues affecting the growth of the plastic surgery biomaterials market are the challenges to plastic surgery and effect on suppliers from the Biomaterials Access Assurance Act.
The European plastic surgery biomaterials market is dominated by Germany. The growth of the European market is expected to be driven by increasing government investments in the biomaterials sector, reimbursements offered by the Centers for Medicare and Medicaid Services (CMS), and the rising aging population, who are the main consumers of plastic surgery biomaterials.
Some of the key players in the plastic surgery biomaterials market include S&V Technologies, Rayner, and InVision Biomedical, among others. The segment and country-specific company shares, news & deals, M&A, segment specific pipeline products, product approvals, and product recalls of the major companies have been detailed in this report.
Customization Options:
Along with the market data, customize the MMM assessments to meet your company’s specific needs. Customize to get a comprehensive summary of the industry standards and a deep dive analysis of the following parameters:
Product Analysis:
- Usage pattern (in-depth trend analysis) of products (segment-wise)
- Product matrix, which gives a detailed comparison of product portfolio of each company, mapped at country and sub-segment levels
- End-user adoption rate analysis of the products (segment-wise and country-wise)
- A comprehensive coverage of product approvals, pipeline products, and product recalls
Epidemiology Data
- Country-specific prevalence of plastic surgery applications of biomaterials
- Country-specific patient pool of plastic surgery applications of biomaterials (pattern analysis)
Procedure Volume Data
- Biomaterials used in plastic surgery procedures performed annually in each country, tracked till sub-segment level
- The number of plastic surgery procedures performed in each country
Surgeons’/Physicians’ Perception Analysis:
- Fast turn-around analysis of surgeons’ responses to market events and trends
- Pattern analysis of usage of biomaterials by physicians
- Surgeons’ opinions about products from different companies
- Surgeons’ qualitative inputs on plastic surgery data
Brand/Product Perception Matrix:
- A comprehensive study of customers’ perception and behavior through our inbuilt social connect tool checking the virality and tonality of blogs
- Analysis of overall brand usage and familiarity and brand advocacy distribution (detractor/neutral/familiar)
Alternative Products: Impact analysis
MMM’s Healthcare Decision Making Quadrant: It is an innovative and useful quadrant for vendors who wish to analyze the potential growth markets based on parameters such as patient dynamics (patient pool, epidemiology of disease, preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and plastic surgery clinics, reimbursement scenario, diagnosis rate, treatment rate, and healthcare expenditure).
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
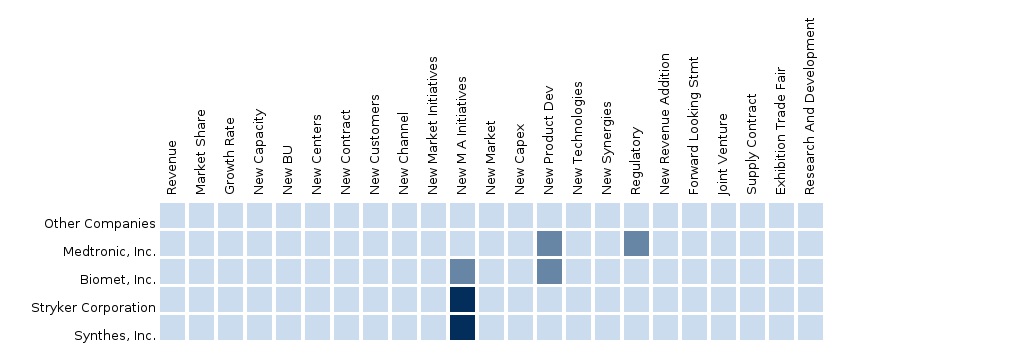
North American Biomaterials Market The report “The North American Biomaterials market forecast 2012-2014” analyzes the market by technology devices into 4 segments such as Metals, Ceramics, Polymers and Natural Biomaterial. Some of the key players in The North American Biomaterials market include Medtronic, Inc., Biomet, Inc., Stryker Corporation, Synthes, Inc. and others. The segment and country-specific company shares, news & deals, M&A, segment specific pipeline products, product approvals, and product recalls of the major companies have been detailed in this report. |
Upcoming |
 |
European Biomaterials Market The report “The European Biomaterials market forecast 2012-2014” analyzes the market by technology devices into 4 segments such as Metals, Ceramics, Polymers and Natural Biomaterial. Some of the key players in The European Biomaterials market include Medtronic, Inc., Biomet, Inc., Stryker Corporation, Synthes, Inc. and others. The segment and country-specific company shares, news & deals, M&A, segment specific pipeline products, product approvals, and product recalls of the major companies have been detailed in this report. |
Upcoming |
 |
Asian Biomaterials Market The report “The Asian Biomaterials market forecast 2012-2014” analyzes the market by technology devices into 4 segments such as Metals, Ceramics, Polymers and Natural Biomaterial. Some of the key players in The Asian Biomaterials market include Medtronic, Inc., Biomet, Inc., Stryker Corporation, Synthes, Inc. and others. The segment and country-specific company shares, news & deals, M&A, segment specific pipeline products, product approvals, and product recalls of the major companies have been detailed in this report. |
Upcoming |












