The European biomaterials market has witnessed dynamic market conditions, and is mainly driven by increased investments, funds & grants by government bodies worldwide, precipitous growth anticipated for plastic surgery and wound healing applications, inflated usage of polymers, growing number of elderly people, technological advancements, and changing lifestyles of the population.
The report ‘The European Biomaterials market forecast 2012-2014’ analyzes the market by technology devices into four segments, such as metals, ceramics, polymers, and natural biomaterial.
Incessant rise in the number of collaborations, conferences, and research-related activities, technological advancements, and increasing applications of biomaterials are the major factors propelling the growth of the biomaterial market in this region. Immunological and inflammatory reactions, stringent regulatory systems, issue of fatigue fracture & wear, and reimbursement concerns are the major restraints curbing the biomaterial market.
The European biomaterials market has witnessed a plethora of growth opportunities. Developments of novel biomaterials for healing wounds, plastic surgery, tissue engineering, ophthalmology, and neurology fuel the growth of this market. The burning issues affecting the growth of biomaterial market are challenges to tissue engineering and effect on suppliers by Biomaterials Access Assurance Act.
The European market growth will be driven by increasing government investments in the biomaterial sector, reimbursements offered by Center for Medicare and Medicaid Services (CMS), and rising aging population who are the main consumers of biomaterials. The European biomaterial market is expected to receive a significant push due to phenomenal increase in new products such as botox, botulinum toxins, and hyaluronic-based injectables.
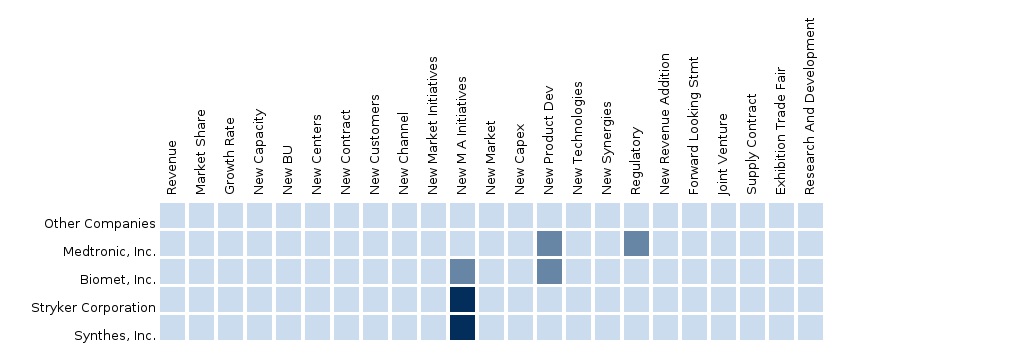
Some of the key players in the European biomaterials market include Medtronic, Inc., Biomet, Inc., Stryker Corporation, Synthes, Inc., and others. The segment and country-specific company shares, news & deals, M&A, segment-specific pipeline products, product approvals, and product recalls of the major companies have been detailed in this report.
Customization Options
Along with the market data, you can also customize the MMM assessments to meet your company’s specific needs. Customize to get a comprehensive summary of the industry standards and a deep-dive analysis of the following parameters:
Product Analysis
- Usage pattern (in-depth trend analysis) of products (segment-wise)
- Product matrix which gives a detailed comparison of product portfolio of each company mapped at country and sub-segment levels
- End-user adoption rate analysis of the products (segment-wise and country-wise)
- A comprehensive coverage of product approvals, pipeline products, and product recalls
Epidemiology Data
- Country-specific prevalence of plastic surgery and wound healing applications
- Country-specific patient pool of plastic surgery and wound healing applications disease (pattern analysis)
Procedure Volume Data
- Plastic surgery and wound healing procedures performed annually in each country tracked till sub-segment level
- The number of plastic surgery and wound healing procedures performed in each country
Surgeons/Physicians Perception Analysis
- Fast turn-around analysis of surgeons’ responses to market events and trends
- Pattern analysis of usage of biomaterials by physicians
- Surgeons’ opinions about products from different companies
- Surgeons’ qualitative inputs on plastic surgery data
Brand/Product Perception Matrix
- Comprehensive study of customers’ perception and behavior through our in-built social connect tool checking the virality and tonality of blogs
- Analysis of overall brand usage and familiarity and brand advocacy distribution (detractor/neutral/familiar)
Alternative Products: Impact analysis
MMM’s healthcare decision making quadrant is an innovative and useful quadrant for vendors who wish to analyze the potential growth markets based on parameters like patient dynamics (patient pool, epidemiology of disease, and preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and orthopedic clinics, reimbursement scenario, diagnosis rate, treatment rate, and healthcare expenditure).
1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Biomaterials-Europe, By Applications
4.1 Split By Geography
4.1 Biomaterials-Spain by Applications
4.1 Biomaterials-United Kingdom by Applications
4.1 Biomaterials-France by Applications
4.1 Biomaterials-Germany by Applications
4.1 Biomaterials-Italy by Applications
4.1 Biomaterials-Rest of Europe by Applications
4.2 Other Biomaterial Applications-Europe
4.2.1 Other Biomaterial Applications-Europe, By Geographies
4.2.1.1 Other Biomaterial Applications-Spain
4.2.1.2 Other Biomaterial Applications-United Kingdom
4.2.1.3 Other Biomaterial Applications-France
4.2.1.4 Other Biomaterial Applications-Germany
4.2.1.5 Other Biomaterial Applications-Italy
4.2.1.6 Other Biomaterial Applications-Rest of Europe
4.2.2 Other Biomaterial Applications-Europe, By Types
4.2.2.1 Drug Delivery Systems Applications-Europe
4.2.2.2 Urinary Applications-Europe
4.2.2.3 Bariatric Surgery Applications-Europe
4.2.2.4 Gastrointestinal Applications-Europe
4.3 Opthalmology-Europe
4.3.1 Opthalmology-Europe, By Companies
4.3.1.1 Opthalmology-Europe-Other Companies
4.3.1.2 Opthalmology-Europe-Medtronic, Inc.
4.3.1.3 Opthalmology-Europe-Biomet, Inc.
4.3.1.4 Opthalmology-Europe-Stryker Corporation
4.3.1.5 Opthalmology-Europe-Synthes, Inc.
4.3.2 Opthalmology-Europe, By MacroIndicators
4.3.2.1 Opthalmology-Healthcare Expenditure-Europe
4.3.3 Opthalmology-Europe, By Geographies
4.3.3.1 Opthalmology-Spain
4.3.3.2 Opthalmology-United Kingdom
4.3.3.3 Opthalmology-France
4.3.3.4 Opthalmology-Germany
4.3.3.5 Opthalmology-Italy
4.3.3.6 Opthalmology-Rest of Europe
4.4 Neurological disorders/Central nervous system-Europe
4.4.1 Neurological disorders/Central nervous system-Europe, By Companies
4.4.1.1 Neurological disorders/Central nervous system-Europe-Other Companies
4.4.1.2 Neurological disorders/Central nervous system-Europe-Medtronic, Inc.
4.4.1.3 Neurological disorders/Central nervous system-Europe-Biomet, Inc.
4.4.1.4 Neurological disorders/Central nervous system-Europe-Stryker Corporation
4.4.1.5 Neurological disorders/Central nervous system-Europe-Synthes, Inc.
4.4.2 Neurological disorders/Central nervous system-Europe, By MacroIndicators
4.4.2.1 Neurological disorders/Central nervous system-Healthcare Expenditure-Europe
4.4.3 Neurological disorders/Central nervous system-Europe, By Geographies
4.4.3.1 Neurological disorders/Central nervous system-Spain
4.4.3.2 Neurological disorders/Central nervous system-United Kingdom
4.4.3.3 Neurological disorders/Central nervous system-France
4.4.3.4 Neurological disorders/Central nervous system-Germany
4.4.3.5 Neurological disorders/Central nervous system-Italy
4.4.3.6 Neurological disorders/Central nervous system-Rest of Europe
4.4.4 Neurological disorders/Central nervous system-Europe, By Products
4.4.4.1 Hydrogel Scaffolds For CNS Repair-Europe
4.4.4.2 Cortical Neural Prosthetics (CNP)-Europe
4.4.4.3 Shunting Systems-Europe
4.4.4.4 Neural Stem Cell Encapsulation-Europe
4.5 Wound Healing-Europe
4.5.1 Wound Healing-Europe, By Companies
4.5.1.1 Wound Healing-Europe-Other Companies
4.5.1.2 Wound Healing-Europe-Medtronic, Inc.
4.5.1.3 Wound Healing-Europe-Biomet, Inc.
4.5.1.4 Wound Healing-Europe-Stryker Corporation
4.5.1.5 Wound Healing-Europe-Synthes, Inc.
4.5.2 Wound Healing-Europe, By MacroIndicators
4.5.2.1 Wound Healing-Healthcare Expenditure-Europe
4.5.3 Wound Healing-Europe, By Geographies
4.5.3.1 Wound Healing-Spain
4.5.3.2 Wound Healing-United Kingdom
4.5.3.3 Wound Healing-France
4.5.3.4 Wound Healing-Germany
4.5.3.5 Wound Healing-Italy
4.5.3.6 Wound Healing-Rest of Europe
4.5.4 Wound Healing-Europe, By Types
4.5.4.1 Adhesion Barrier-Europe
4.5.4.2 Fracture Fixation Devices-Europe
4.5.4.3 Skin Substitutes-Europe
4.5.4.4 Surgical Hemostats-Europe
4.5.4.5 Internal Tissue Sealant-Europe
4.6 Tissue Regeneration-Europe
4.6.1 Tissue Regeneration-Europe, By Companies
4.6.1.1 Tissue Regeneration-Europe-Other Companies
4.6.1.2 Tissue Regeneration-Europe-Medtronic, Inc.
4.6.1.3 Tissue Regeneration-Europe-Biomet, Inc.
4.6.1.4 Tissue Regeneration-Europe-Stryker Corporation
4.6.1.5 Tissue Regeneration-Europe-Synthes, Inc.
4.6.2 Tissue Regeneration-Europe, By MacroIndicators
4.6.2.1 Tissue Regeneration-Healthcare Expenditure-Europe
4.6.3 Tissue Regeneration-Europe, By Geographies
4.6.3.1 Tissue Regeneration-Spain
4.6.3.2 Tissue Regeneration-United Kingdom
4.6.3.3 Tissue Regeneration-France
4.6.3.4 Tissue Regeneration-Germany
4.6.3.5 Tissue Regeneration-Italy
4.6.3.6 Tissue Regeneration-Rest of Europe
4.6.4 Tissue Regeneration-Europe, By Products
4.6.4.1 Scaffolds for Regenerative Medicine-Europe
4.6.4.2 Tailoring of Inorganic Nano-particles (gold and quantum dots with bio-active pep tides)-Europe
4.6.4.3 Self-assembled Bio-mimetic Co-polymers-Europe
4.6.4.4 Nano-materials for Bio-sensing Applications-Europe
4.6.4.5 Cell-materials Interface Engineering-Europe
4.7 Orthopedic-Europe
4.7.1 Orthopedic-Europe, By Companies
4.7.1.1 Orthopedic-Europe-Other Companies
4.7.1.2 Orthopedic-Europe-Medtronic, Inc.
4.7.1.3 Orthopedic-Europe-Biomet, Inc.
4.7.1.4 Orthopedic-Europe-Stryker Corporation
4.7.1.5 Orthopedic-Europe-Synthes, Inc.
4.7.2 Orthopedic-Europe, By MacroIndicators
4.7.2.1 Orthopedic-Healthcare Expenditure-Europe
4.7.3 Orthopedic-Europe, By Geographies
4.7.3.1 Orthopedic-Spain
4.7.3.2 Orthopedic-United Kingdom
4.7.3.3 Orthopedic-France
4.7.3.4 Orthopedic-Germany
4.7.3.5 Orthopedic-Italy
4.7.3.6 Orthopedic-Rest of Europe
4.7.4 Orthopedic-Europe, By Types
4.7.4.1 Spine Biomaterial-Europe
4.7.4.2 Orthobiologics biomaterials-Europe
4.7.4.3 Viscosupplementation-Europe
4.7.4.4 Bioresorbable Tissue Fixation Products-Europe
4.7.4.5 Joint Replacement/Reconstruction-Europe
4.8 Dental-Europe
4.8.1 Dental-Europe, By Companies
4.8.1.1 Dental-Europe-Other Companies
4.8.1.2 Dental-Europe-Medtronic, Inc.
4.8.1.3 Dental-Europe-Biomet, Inc.
4.8.1.4 Dental-Europe-Stryker Corporation
4.8.1.5 Dental-Europe-Synthes, Inc.
4.8.2 Dental-Europe, By MacroIndicators
4.8.2.1 Dental-Healthcare Expenditure-Europe
4.8.3 Dental-Europe, By Geographies
4.8.3.1 Dental-Spain
4.8.3.2 Dental-United Kingdom
4.8.3.3 Dental-France
4.8.3.4 Dental-Germany
4.8.3.5 Dental-Italy
4.8.3.6 Dental-Rest of Europe
4.8.4 Dental-Europe, By Products
4.8.4.1 Dental Bone Graft & Substitutes-Europe
4.8.4.2 Dental Membranes-Europe
4.8.4.3 Dental Implants-Europe
4.8.4.4 Tissue Regeneration Materials-Europe
4.9 Plastic Surgery (biomaterials)-Europe
4.9.1 Plastic Surgery (biomaterials)-Europe, By Companies
4.9.1.1 Plastic Surgery (biomaterials)-Europe-Other Companies
4.9.1.2 Plastic Surgery (biomaterials)-Europe-Medtronic, Inc.
4.9.1.3 Plastic Surgery (biomaterials)-Europe-Biomet, Inc.
4.9.1.4 Plastic Surgery (biomaterials)-Europe-Stryker Corporation
4.9.1.5 Plastic Surgery (biomaterials)-Europe-Synthes, Inc.
4.9.2 Plastic Surgery (biomaterials)-Europe, By MacroIndicators
4.9.2.1 Plastic Surgery (biomaterials)-Healthcare Expenditure-Europe
4.9.3 Plastic Surgery (biomaterials)-Europe, By Geographies
4.9.3.1 Plastic Surgery (biomaterials)-Spain
4.9.3.2 Plastic Surgery (biomaterials)-United Kingdom
4.9.3.3 Plastic Surgery (biomaterials)-France
4.9.3.4 Plastic Surgery (biomaterials)-Germany
4.9.3.5 Plastic Surgery (biomaterials)-Italy
4.9.3.6 Plastic Surgery (biomaterials)-Rest of Europe
4.9.4 Plastic Surgery (biomaterials)-Europe, By Products
4.9.4.1 Craniofacial Surgery-Europe
4.9.4.2 Soft Tissue Fillers-Europe
4.9.4.3 Acellular Dermal Matrices-Europe
4.9.4.4 Peripheral Nerve Repair-Europe
4.9.4.5 Facial Wrinkle Treatment (Hyaluronic Acid Based)-Europe
4.9.4.6 Bio engineered Skins-Europe
4.10 Cardiovascular applications-Europe
4.10.1 Cardiovascular applications-Europe, By Companies
4.10.1.1 Cardiovascular applications-Europe-Other Companies
4.10.1.2 Cardiovascular applications-Europe-Medtronic, Inc.
4.10.1.3 Cardiovascular applications-Europe-Biomet, Inc.
4.10.1.4 Cardiovascular applications-Europe-Stryker Corporation
4.10.1.5 Cardiovascular applications-Europe-Synthes, Inc.
4.10.2 Cardiovascular applications-Europe, By MacroIndicators
4.10.2.1 Cardiovascular applications-Healthcare Expenditure-Europe
4.10.3 Cardiovascular applications-Europe, By Geographies
4.10.3.1 Cardiovascular applications-Spain
4.10.3.2 Cardiovascular applications-United Kingdom
4.10.3.3 Cardiovascular applications-France
4.10.3.4 Cardiovascular applications-Germany
4.10.3.5 Cardiovascular applications-Italy
4.10.3.6 Cardiovascular applications-Rest of Europe
4.10.4 Cardiovascular applications-Europe, By Products
4.10.4.1 Cardiac Sensors-Europe
4.10.4.2 Cardiac Catheters-Europe
4.10.4.3 Others-Extracorporeal Oxygenation, Artificial Kidney (hemodialyzer), Ventricular Assist Device (VAD), Sternum Closure Devices, Introducer Sheaths-Europe
4.10.4.4 Cardiac Stents-Europe
4.10.4.5 Cardiac Guidewires-Europe
4.10.4.6 Cardiac Vascular Grafts-Europe
4.10.4.7 Heart Valves-Europe
4.10.4.8 Implantable Cardiac Defibrillators-Europe
4.10.4.9 Cardiac Pacemakers-Europe
5 Biomaterials-Europe, By Ingredients
5.1 Split By Geography
5.2 Biomaterials-Spain by Ingredients
5.1 Biomaterials-United Kingdom by Ingredients
5.1 Biomaterials-France by Ingredients
5.1 Biomaterials-Germany by Ingredients
5.1 Biomaterials-Italy by Ingredients
5.1 Biomaterials-Rest of Europe by Ingredients
5.2 Biomaterials-Polyhydroxyalkanoate (PHA)-Europe
5.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Europe, By Geographies
5.2.1.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Spain
5.2.1.2 Biomaterials-Polyhydroxyalkanoate (PHA)-United Kingdom
5.2.1.3 Biomaterials-Polyhydroxyalkanoate (PHA)-France
5.2.1.4 Biomaterials-Polyhydroxyalkanoate (PHA)-Germany
5.2.1.5 Biomaterials-Polyhydroxyalkanoate (PHA)-Italy
5.2.1.6 Biomaterials-Polyhydroxyalkanoate (PHA)-Rest of Europe
5.3 Biomaterials-Silicone-Europe
5.3.1 Biomaterials-Silicone-Europe, By Geographies
5.3.1.1 Biomaterials-Silicone-Spain
5.3.1.2 Biomaterials-Silicone-United Kingdom
5.3.1.3 Biomaterials-Silicone-France
5.3.1.4 Biomaterials-Silicone-Germany
5.3.1.5 Biomaterials-Silicone-Italy
5.3.1.6 Biomaterials-Silicone-Rest of Europe
5.4 Biomaterials-Silicones-Europe
5.4.1 Biomaterials-Silicones-Europe, By Ingredients
5.4.1.1 Biomaterials-Silicone-Europe
5.4.2 Biomaterials-Silicones-Europe, By Geographies
5.4.2.1 Biomaterials-Silicones-Spain
5.4.2.2 Biomaterials-Silicones-United Kingdom
5.4.2.3 Biomaterials-Silicones-France
5.4.2.4 Biomaterials-Silicones-Germany
5.4.2.5 Biomaterials-Silicones-Italy
5.4.2.6 Biomaterials-Silicones-Rest of Europe
5.5 Biomaterials-Dispersants-Europe
5.5.1 Biomaterials-Dispersants-Europe, By Geographies
5.5.1.1 Biomaterials-Dispersants-Spain
5.5.1.2 Biomaterials-Dispersants-United Kingdom
5.5.1.3 Biomaterials-Dispersants-France
5.5.1.4 Biomaterials-Dispersants-Germany
5.5.1.5 Biomaterials-Dispersants-Italy
5.5.1.6 Biomaterials-Dispersants-Rest of Europe
5.6 Biomaterials-Polyether Ether Ketone (PEEK)-Europe
5.6.1 Biomaterials-Polyether Ether Ketone (PEEK)-Europe, By Geographies
5.6.1.1 Biomaterials-Polyether Ether Ketone (PEEK)-Spain
5.6.1.2 Biomaterials-Polyether Ether Ketone (PEEK)-United Kingdom
5.6.1.3 Biomaterials-Polyether Ether Ketone (PEEK)-France
5.6.1.4 Biomaterials-Polyether Ether Ketone (PEEK)-Germany
5.6.1.5 Biomaterials-Polyether Ether Ketone (PEEK)-Italy
5.6.1.6 Biomaterials-Polyether Ether Ketone (PEEK)-Rest of Europe
5.7 Biomaterials-Microsphere-Europe
5.7.1 Biomaterials-Microsphere-Europe, By Geographies
5.7.1.1 Biomaterials-Microsphere-Spain
5.7.1.2 Biomaterials-Microsphere-United Kingdom
5.7.1.3 Biomaterials-Microsphere-France
5.7.1.4 Biomaterials-Microsphere-Germany
5.7.1.5 Biomaterials-Microsphere-Italy
5.7.1.6 Biomaterials-Microsphere-Rest of Europe
6 Biomaterials-Europe, By Types
6.1 Split By Geography
6.3 Biomaterials-Spain by Types
6.1 Biomaterials-United Kingdom by Types
6.1 Biomaterials-France by Types
6.1 Biomaterials-Germany by Types
6.1 Biomaterials-Italy by Types
6.1 Biomaterials-Rest of Europe by Types
6.2 Metallic Biomaterials-Europe
6.2.1 Metallic Biomaterials-Europe, By Types
6.2.1.1 Metallic Biomaterials-Stainless Steel-Europe
6.2.1.2 Metallic Biomaterials-Titanium Alloys-Europe
6.2.1.3 Metallic Biomaterials-Silver-Europe
6.2.1.4 Metallic Biomaterials-Cobalt-Chrome Alloys-Europe
6.2.1.5 Metallic Biomaterials-Gold-Europe
6.2.1.6 Metallic Biomaterials-Platinum-Europe
6.2.2 Metallic Biomaterials-Europe, By Geographies
6.2.2.1 Metallic Biomaterials-Spain
6.2.2.2 Metallic Biomaterials-United Kingdom
6.2.2.3 Metallic Biomaterials-France
6.2.2.4 Metallic Biomaterials-Germany
6.2.2.5 Metallic Biomaterials-Italy
6.2.2.6 Metallic Biomaterials-Rest of Europe
6.2.3 Metallic Biomaterials-Europe, By Materials
6.2.3.1 Cobalt-Chrome Alloys-Europe
6.2.3.2 Gold-Europe
6.2.3.3 Stainless Steel-Europe
6.2.3.4 Titanium & Titanium Alloys-Europe
6.2.3.5 Platinum-Europe
6.2.3.6 Silver-Europe
6.3 Ceramics Biomaterials-Europe
6.3.1 Ceramics Biomaterials-Europe, By Types
6.3.1.1 Ceramics Biomaterials-Calcium Phosphate-Europe
6.3.1.2 Ceramics Biomaterials-Zirconium Dioxide OR Zirconia-Europe
6.3.1.3 Ceramics Biomaterials-Aluminium Hydroxide OR Aluminum Trihydrate (ATH)-Europe
6.3.1.4 Ceramics Biomaterials-Carbon-Europe
6.3.1.5 Ceramics Biomaterials-Calcium Sulphate-Europe
6.3.1.6 Ceramics Biomaterials-Glass-Europe
6.3.2 Ceramics Biomaterials-Europe, By Geographies
6.3.2.1 Ceramics Biomaterials-Spain
6.3.2.2 Ceramics Biomaterials-United Kingdom
6.3.2.3 Ceramics Biomaterials-France
6.3.2.4 Ceramics Biomaterials-Germany
6.3.2.5 Ceramics Biomaterials-Italy
6.3.2.6 Ceramics Biomaterials-Rest of Europe
6.3.3 Ceramics Biomaterials-Europe, By Materials
6.3.3.1 Carbon-Europe
6.3.3.2 Calcium Sulfate-Europe
6.3.3.3 Calcium Phosphate-Europe
6.3.3.4 Zirconia-Europe
6.3.3.5 Aluminium Oxide-Europe
6.3.3.6 Glass-Europe
6.4 Polymer Biomaterials-Europe
6.4.1 Polymer Biomaterials-Europe, By Types
6.4.1.1 Polymer Biomaterials-Polymethyl Methacrylate (PMMA)-Europe
6.4.1.2 Polymer Biomaterials-Polyethylene (PE)-Europe
6.4.1.3 Polymer Biomaterials-Polyester-Europe
6.4.1.4 Polymer Biomaterials-Silicone Rubber-Europe
6.4.1.5 Polymer Biomaterials-Polyvinyl Chloride Resins-Europe
6.4.1.6 Polymer Biomaterials-Nylon-Europe
6.4.2 Polymer Biomaterials-Europe, By Geographies
6.4.2.1 Polymer Biomaterials-Spain
6.4.2.2 Polymer Biomaterials-United Kingdom
6.4.2.3 Polymer Biomaterials-France
6.4.2.4 Polymer Biomaterials-Germany
6.4.2.5 Polymer Biomaterials-Italy
6.4.2.6 Polymer Biomaterials-Rest of Europe
6.4.3 Polymer Biomaterials-Europe, By Materials
6.4.3.1 Polymethylmethacrylate (PMMA)-Europe
6.4.3.2 Polyvinyl Chloride-Europe
6.4.3.3 Silicone Rubber-Europe
6.4.3.4 Polyethylene-Europe
6.4.3.5 Nylon-Europe
6.4.3.6 Polyester-Europe
6.5 Natural Biomaterials-Europe
6.5.1 Natural Biomaterials-Europe, By Types
6.5.1.1 Natural Biomaterials-Hyaluronic Acid-Europe
6.5.1.2 Natural Biomaterials-Cellulose-Europe
6.5.1.3 Natural Biomaterials-Chitin-Europe
6.5.1.4 Natural Biomaterials-Alginates-Europe
6.5.2 Natural Biomaterials-Europe, By Geographies
6.5.2.1 Natural Biomaterials-Spain
6.5.2.2 Natural Biomaterials-United Kingdom
6.5.2.3 Natural Biomaterials-France
6.5.2.4 Natural Biomaterials-Germany
6.5.2.5 Natural Biomaterials-Italy
6.5.2.6 Natural Biomaterials-Rest of Europe
6.5.3 Natural Biomaterials-Europe, By Materials
6.5.3.1 Collagen & Gelatin-Europe
6.5.3.2 Hyaluronic Acid-Europe
6.5.3.3 Alginate-Europe
6.5.3.4 Chitin-Europe
6.5.3.5 Cellulose-Europe
7 Biomaterials-Europe, By Geographies
7.1 Biomaterials-Spain
7.1.1 Biomaterials-Spain, By Companies
7.1.1.1 Biomaterials-Spain-Other Companies
7.1.1.2 Biomaterials-Spain-Medtronic, Inc.
7.1.1.3 Biomaterials-Spain-Biomet, Inc.
7.1.1.4 Biomaterials-Spain-Stryker Corporation
7.1.1.5 Biomaterials-Spain-Synthes, Inc.
7.1.2 Biomaterials-Spain, By Ingredients
7.1.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Spain
7.1.2.2 Biomaterials-Silicone-Spain
7.1.2.3 Biomaterials-Silicones-Spain
7.1.2.4 Biomaterials-Dispersants-Spain
7.1.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-Spain
7.1.2.6 Biomaterials-Microsphere-Spain
7.1.3 Biomaterials-Spain, By Applications
7.1.3.1 Other Biomaterial Applications-Spain
7.1.3.2 Opthalmology-Spain
7.1.3.3 Neurological disorders/Central nervous system-Spain
7.1.3.4 Wound Healing-Spain
7.1.3.5 Tissue Regeneration-Spain
7.1.3.6 Orthopedic-Spain
7.1.3.7 Dental-Spain
7.1.3.8 Plastic Surgery (biomaterials)-Spain
7.1.3.9 Cardiovascular applications-Spain
7.1.4 Biomaterials-Spain, By Types
7.1.4.1 Metallic Biomaterials-Spain
7.1.4.2 Ceramics Biomaterials-Spain
7.1.4.3 Polymer Biomaterials-Spain
7.1.4.4 Natural Biomaterials-Spain
7.2 Biomaterials-United Kingdom
7.2.1 Biomaterials-United Kingdom, By Companies
7.2.1.1 Biomaterials-United Kingdom-Other Companies
7.2.1.2 Biomaterials-United Kingdom-Medtronic, Inc.
7.2.1.3 Biomaterials-United Kingdom-Biomet, Inc.
7.2.1.4 Biomaterials-United Kingdom-Stryker Corporation
7.2.1.5 Biomaterials-United Kingdom-Synthes, Inc.
7.2.2 Biomaterials-United Kingdom, By Ingredients
7.2.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-United Kingdom
7.2.2.2 Biomaterials-Silicone-United Kingdom
7.2.2.3 Biomaterials-Silicones-United Kingdom
7.2.2.4 Biomaterials-Dispersants-United Kingdom
7.2.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-United Kingdom
7.2.2.6 Biomaterials-Microsphere-United Kingdom
7.2.3 Biomaterials-United Kingdom, By Applications
7.2.3.1 Other Biomaterial Applications-United Kingdom
7.2.3.2 Opthalmology-United Kingdom
7.2.3.3 Neurological disorders/Central nervous system-United Kingdom
7.2.3.4 Wound Healing-United Kingdom
7.2.3.5 Tissue Regeneration-United Kingdom
7.2.3.6 Orthopedic-United Kingdom
7.2.3.7 Dental-United Kingdom
7.2.3.8 Plastic Surgery (biomaterials)-United Kingdom
7.2.3.9 Cardiovascular applications-United Kingdom
7.2.4 Biomaterials-United Kingdom, By Types
7.2.4.1 Metallic Biomaterials-United Kingdom
7.2.4.2 Ceramics Biomaterials-United Kingdom
7.2.4.3 Polymer Biomaterials-United Kingdom
7.2.4.4 Natural Biomaterials-United Kingdom
7.3 Biomaterials-France
7.3.1 Biomaterials-France, By Companies
7.3.1.1 Biomaterials-France-Other Companies
7.3.1.2 Biomaterials-France-Medtronic, Inc.
7.3.1.3 Biomaterials-France-Biomet, Inc.
7.3.1.4 Biomaterials-France-Stryker Corporation
7.3.1.5 Biomaterials-France-Synthes, Inc.
7.3.2 Biomaterials-France, By Ingredients
7.3.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-France
7.3.2.2 Biomaterials-Silicone-France
7.3.2.3 Biomaterials-Silicones-France
7.3.2.4 Biomaterials-Dispersants-France
7.3.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-France
7.3.2.6 Biomaterials-Microsphere-France
7.3.3 Biomaterials-France, By Applications
7.3.3.1 Other Biomaterial Applications-France
7.3.3.2 Opthalmology-France
7.3.3.3 Neurological disorders/Central nervous system-France
7.3.3.4 Wound Healing-France
7.3.3.5 Tissue Regeneration-France
7.3.3.6 Orthopedic-France
7.3.3.7 Dental-France
7.3.3.8 Plastic Surgery (biomaterials)-France
7.3.3.9 Cardiovascular applications-France
7.3.4 Biomaterials-France, By Types
7.3.4.1 Metallic Biomaterials-France
7.3.4.2 Ceramics Biomaterials-France
7.3.4.3 Polymer Biomaterials-France
7.3.4.4 Natural Biomaterials-France
7.4 Biomaterials-Germany
7.4.1 Biomaterials-Germany, By Companies
7.4.1.1 Biomaterials-Germany-Other Companies
7.4.1.2 Biomaterials-Germany-Medtronic, Inc.
7.4.1.3 Biomaterials-Germany-Biomet, Inc.
7.4.1.4 Biomaterials-Germany-Stryker Corporation
7.4.1.5 Biomaterials-Germany-Synthes, Inc.
7.4.2 Biomaterials-Germany, By Ingredients
7.4.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Germany
7.4.2.2 Biomaterials-Silicone-Germany
7.4.2.3 Biomaterials-Silicones-Germany
7.4.2.4 Biomaterials-Dispersants-Germany
7.4.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-Germany
7.4.2.6 Biomaterials-Microsphere-Germany
7.4.3 Biomaterials-Germany, By Applications
7.4.3.1 Other Biomaterial Applications-Germany
7.4.3.2 Opthalmology-Germany
7.4.3.3 Neurological disorders/Central nervous system-Germany
7.4.3.4 Wound Healing-Germany
7.4.3.5 Tissue Regeneration-Germany
7.4.3.6 Orthopedic-Germany
7.4.3.7 Dental-Germany
7.4.3.8 Plastic Surgery (biomaterials)-Germany
7.4.3.9 Cardiovascular applications-Germany
7.4.4 Biomaterials-Germany, By Types
7.4.4.1 Metallic Biomaterials-Germany
7.4.4.2 Ceramics Biomaterials-Germany
7.4.4.3 Polymer Biomaterials-Germany
7.4.4.4 Natural Biomaterials-Germany
7.5 Biomaterials-Italy
7.5.1 Biomaterials-Italy, By Companies
7.5.1.1 Biomaterials-Italy-Other Companies
7.5.1.2 Biomaterials-Italy-Medtronic, Inc.
7.5.1.3 Biomaterials-Italy-Biomet, Inc.
7.5.1.4 Biomaterials-Italy-Stryker Corporation
7.5.1.5 Biomaterials-Italy-Synthes, Inc.
7.5.2 Biomaterials-Italy, By Ingredients
7.5.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Italy
7.5.2.2 Biomaterials-Silicone-Italy
7.5.2.3 Biomaterials-Silicones-Italy
7.5.2.4 Biomaterials-Dispersants-Italy
7.5.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-Italy
7.5.2.6 Biomaterials-Microsphere-Italy
7.5.3 Biomaterials-Italy, By Applications
7.5.3.1 Other Biomaterial Applications-Italy
7.5.3.2 Opthalmology-Italy
7.5.3.3 Neurological disorders/Central nervous system-Italy
7.5.3.4 Wound Healing-Italy
7.5.3.5 Tissue Regeneration-Italy
7.5.3.6 Orthopedic-Italy
7.5.3.7 Dental-Italy
7.5.3.8 Plastic Surgery (biomaterials)-Italy
7.5.3.9 Cardiovascular applications-Italy
7.5.4 Biomaterials-Italy, By Types
7.5.4.1 Metallic Biomaterials-Italy
7.5.4.2 Ceramics Biomaterials-Italy
7.5.4.3 Polymer Biomaterials-Italy
7.5.4.4 Natural Biomaterials-Italy
7.6 Biomaterials-Rest of Europe
7.6.1 Biomaterials-Rest of Europe, By Companies
7.6.1.1 Biomaterials-Rest of Europe-Other Companies
7.6.1.2 Biomaterials-Rest of Europe-Medtronic, Inc.
7.6.1.3 Biomaterials-Rest of Europe-Biomet, Inc.
7.6.1.4 Biomaterials-Rest of Europe-Stryker Corporation
7.6.1.5 Biomaterials-Rest of Europe-Synthes, Inc.
7.6.2 Biomaterials-Rest of Europe, By Ingredients
7.6.2.1 Biomaterials-Polyhydroxyalkanoate (PHA)-Rest of Europe
7.6.2.2 Biomaterials-Silicone-Rest of Europe
7.6.2.3 Biomaterials-Silicones-Rest of Europe
7.6.2.4 Biomaterials-Dispersants-Rest of Europe
7.6.2.5 Biomaterials-Polyether Ether Ketone (PEEK)-Rest of Europe
7.6.2.6 Biomaterials-Microsphere-Rest of Europe
7.6.3 Biomaterials-Rest of Europe, By Applications
7.6.3.1 Other Biomaterial Applications-Rest of Europe
7.6.3.2 Opthalmology-Rest of Europe
7.6.3.3 Neurological disorders/Central nervous system-Rest of Europe
7.6.3.4 Wound Healing-Rest of Europe
7.6.3.5 Tissue Regeneration-Rest of Europe
7.6.3.6 Orthopedic-Rest of Europe
7.6.3.7 Dental-Rest of Europe
7.6.3.8 Plastic Surgery (biomaterials)-Rest of Europe
7.6.3.9 Cardiovascular applications-Rest of Europe
7.6.4 Biomaterials-Rest of Europe, By Types
7.6.4.1 Metallic Biomaterials-Rest of Europe
7.6.4.2 Ceramics Biomaterials-Rest of Europe
7.6.4.3 Polymer Biomaterials-Rest of Europe
7.6.4.4 Natural Biomaterials-Rest of Europe
8 Biomaterials-Europe, By Companies
8.1 Competitive landscape
8.2 Split By Geography
8.4 Biomaterials-Spain by Companies
8.1 Biomaterials-United Kingdom by Companies
8.1 Biomaterials-France by Companies
8.1 Biomaterials-Germany by Companies
8.1 Biomaterials-Italy by Companies
8.1 Biomaterials-Rest of Europe by Companies
8.3 Biomaterials-Europe-Other Companies
8.3.1 Biomaterials-Europe-Other Companies, By Geographies
8.3.1.1 Biomaterials-Spain-Other Companies
8.3.1.2 Biomaterials-United Kingdom-Other Companies
8.3.1.3 Biomaterials-France-Other Companies
8.3.1.4 Biomaterials-Germany-Other Companies
8.3.1.5 Biomaterials-Italy-Other Companies
8.3.1.6 Biomaterials-Rest of Europe-Other Companies
8.3.2 Biomaterials-Europe-Other Companies, By Applications
8.3.2.1 Neurological disorders/Central nervous system-Europe-Other Companies
8.3.2.2 Wound Healing-Europe-Other Companies
8.3.2.3 Plastic Surgery (biomaterials)-Europe-Other Companies
8.3.2.4 Cardiovascular applications-Europe-Other Companies
8.3.2.5 Tissue Regeneration-Europe-Other Companies
8.3.2.6 Orthopedic-Europe-Other Companies
8.3.2.7 Dental-Europe-Other Companies
8.3.2.8 Opthalmology-Europe-Other Companies
8.3.3 Biomaterials-Europe-Other Companies, By Types
8.3.3.1 Metallic Biomaterials-Europe-Other Companies
8.3.3.2 Ceramics Biomaterials-Europe-Other Companies
8.3.3.3 Polymer Biomaterials-Europe-Other Companies
8.3.3.4 Natural Biomaterials-Europe-Other Companies
8.4 Biomaterials-Europe-Medtronic, Inc.
8.4.1 Biomaterials-Europe-Medtronic, Inc., By Geographies
8.4.1.1 Biomaterials-Spain-Medtronic, Inc.
8.4.1.2 Biomaterials-United Kingdom-Medtronic, Inc.
8.4.1.3 Biomaterials-France-Medtronic, Inc.
8.4.1.4 Biomaterials-Germany-Medtronic, Inc.
8.4.1.5 Biomaterials-Italy-Medtronic, Inc.
8.4.1.6 Biomaterials-Rest of Europe-Medtronic, Inc.
8.4.2 Biomaterials-Europe-Medtronic, Inc., By Applications
8.4.2.1 Neurological disorders/Central nervous system-Europe-Medtronic, Inc.
8.4.2.2 Wound Healing-Europe-Medtronic, Inc.
8.4.2.3 Plastic Surgery (biomaterials)-Europe-Medtronic, Inc.
8.4.2.4 Cardiovascular applications-Europe-Medtronic, Inc.
8.4.2.5 Tissue Regeneration-Europe-Medtronic, Inc.
8.4.2.6 Orthopedic-Europe-Medtronic, Inc.
8.4.2.7 Dental-Europe-Medtronic, Inc.
8.4.2.8 Opthalmology-Europe-Medtronic, Inc.
8.4.3 Biomaterials-Europe-Medtronic, Inc., By Types
8.4.3.1 Metallic Biomaterials-Europe-Medtronic, Inc.
8.4.3.2 Ceramics Biomaterials-Europe-Medtronic, Inc.
8.4.3.3 Polymer Biomaterials-Europe-Medtronic, Inc.
8.4.3.4 Natural Biomaterials-Europe-Medtronic, Inc.
8.5 Biomaterials-Europe-Biomet, Inc.
8.5.1 Biomaterials-Europe-Biomet, Inc., By Geographies
8.5.1.1 Biomaterials-Spain-Biomet, Inc.
8.5.1.2 Biomaterials-United Kingdom-Biomet, Inc.
8.5.1.3 Biomaterials-France-Biomet, Inc.
8.5.1.4 Biomaterials-Germany-Biomet, Inc.
8.5.1.5 Biomaterials-Italy-Biomet, Inc.
8.5.1.6 Biomaterials-Rest of Europe-Biomet, Inc.
8.5.2 Biomaterials-Europe-Biomet, Inc., By Applications
8.5.2.1 Neurological disorders/Central nervous system-Europe-Biomet, Inc.
8.5.2.2 Wound Healing-Europe-Biomet, Inc.
8.5.2.3 Plastic Surgery (biomaterials)-Europe-Biomet, Inc.
8.5.2.4 Cardiovascular applications-Europe-Biomet, Inc.
8.5.2.5 Tissue Regeneration-Europe-Biomet, Inc.
8.5.2.6 Orthopedic-Europe-Biomet, Inc.
8.5.2.7 Dental-Europe-Biomet, Inc.
8.5.2.8 Opthalmology-Europe-Biomet, Inc.
8.5.3 Biomaterials-Europe-Biomet, Inc., By Types
8.5.3.1 Metallic Biomaterials-Europe-Biomet, Inc.
8.5.3.2 Ceramics Biomaterials-Europe-Biomet, Inc.
8.5.3.3 Polymer Biomaterials-Europe-Biomet, Inc.
8.5.3.4 Natural Biomaterials-Europe-Biomet, Inc.
8.6 Biomaterials-Europe-Stryker Corporation
8.6.1 Biomaterials-Europe-Stryker Corporation, By Geographies
8.6.1.1 Biomaterials-Spain-Stryker Corporation
8.6.1.2 Biomaterials-United Kingdom-Stryker Corporation
8.6.1.3 Biomaterials-France-Stryker Corporation
8.6.1.4 Biomaterials-Germany-Stryker Corporation
8.6.1.5 Biomaterials-Italy-Stryker Corporation
8.6.1.6 Biomaterials-Rest of Europe-Stryker Corporation
8.6.2 Biomaterials-Europe-Stryker Corporation, By Applications
8.6.2.1 Neurological disorders/Central nervous system-Europe-Stryker Corporation
8.6.2.2 Wound Healing-Europe-Stryker Corporation
8.6.2.3 Plastic Surgery (biomaterials)-Europe-Stryker Corporation
8.6.2.4 Cardiovascular applications-Europe-Stryker Corporation
8.6.2.5 Tissue Regeneration-Europe-Stryker Corporation
8.6.2.6 Orthopedic-Europe-Stryker Corporation
8.6.2.7 Dental-Europe-Stryker Corporation
8.6.2.8 Opthalmology-Europe-Stryker Corporation
8.6.3 Biomaterials-Europe-Stryker Corporation, By Types
8.6.3.1 Metallic Biomaterials-Europe-Stryker Corporation
8.6.3.2 Ceramics Biomaterials-Europe-Stryker Corporation
8.6.3.3 Polymer Biomaterials-Europe-Stryker Corporation
8.6.3.4 Natural Biomaterials-Europe-Stryker Corporation
8.7 Biomaterials-Europe-Synthes, Inc.
8.7.1 Biomaterials-Europe-Synthes, Inc., By Geographies
8.7.1.1 Biomaterials-Spain-Synthes, Inc.
8.7.1.2 Biomaterials-United Kingdom-Synthes, Inc.
8.7.1.3 Biomaterials-France-Synthes, Inc.
8.7.1.4 Biomaterials-Germany-Synthes, Inc.
8.7.1.5 Biomaterials-Italy-Synthes, Inc.
8.7.1.6 Biomaterials-Rest of Europe-Synthes, Inc.
8.7.2 Biomaterials-Europe-Synthes, Inc., By Applications
8.7.2.1 Neurological disorders/Central nervous system-Europe-Synthes, Inc.
8.7.2.2 Wound Healing-Europe-Synthes, Inc.
8.7.2.3 Plastic Surgery (biomaterials)-Europe-Synthes, Inc.
8.7.2.4 Cardiovascular applications-Europe-Synthes, Inc.
8.7.2.5 Tissue Regeneration-Europe-Synthes, Inc.
8.7.2.6 Orthopedic-Europe-Synthes, Inc.
8.7.2.7 Dental-Europe-Synthes, Inc.
8.7.2.8 Opthalmology-Europe-Synthes, Inc.
8.7.3 Biomaterials-Europe-Synthes, Inc., By Types
8.7.3.1 Metallic Biomaterials-Europe-Synthes, Inc.
8.7.3.2 Ceramics Biomaterials-Europe-Synthes, Inc.
8.7.3.3 Polymer Biomaterials-Europe-Synthes, Inc.
8.7.3.4 Natural Biomaterials-Europe-Synthes, Inc.
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
North American BioProduction Market The North American bio-production Market, mainly driven by increasing in-vitro research activities, increasing demand for vaccines and cellular medicine. The report “North American Bio-Production Market forecast, 2008-2018 “analyzes the market by 5 main segments such as tissue engineering, cell culture, protein engineering, biomaterials, and micro-reactor. Geographically, the market is mainly segmented into U.S., Canada, and Mexico. The report also provides an extensive competitive landscaping of companies operating in this market. |
Upcoming |
 |
European BioProduction Market The European BioProduction Market, mainly driven by increasing in-vitro research activities, increasing demand for vaccines and cellular medicine. The report “European BioProduction Market forecast, 2008-2018 “analyzes the market by 5 main segments such as tissue engineering, cell culture, protein engineering, biomaterials, and micro-reactor. Geographically, the European BioProduction Market is mainly segmented into U.K., Germany, France, Italy, Spain, and Rest of Europe. The report European BioProduction Market also provides an extensive competitive landscaping of companies operating in this market. |
Upcoming |












