
North America Spine Surgery Devices Market by Product (Non-Fusion Implants, Spinal Fusion and Fixation Devices, Spine Biologics, Spine Bone Stimulators, VCFT Devices), by End User (Hospitals, Orthopedic Clinics), by Geography - Analysis and Forecast to 2019
North America SPINE SURGERY DEVICES MARKET: By Type (Spinal Fusion & Spinal Non-Fusion), By End-User (Hospitals, Ambulatory Surgical Centers & Orthopedic Clinics), By Region - Forecasts to 2020.
Spine Surgeries are basically recommended to the patients suffering from chronic back pain due to disc herniation; isthmic spondylolisthesis; degenerative diseases, such as degenerative spondylolisthesis, and spinal stenosis; and many others. Generally, low back pain is a common symptom reported by 30.0% patients with spine instability, but the source of pain needs to be identified before undergoing any surgery.
By type, this market is segmented into spinal fusion and spinal non-fusion, wherein spinal fusion, which fuses vertebrae together to treat back pain and is commonly done in the lumbar region of the lower back. The spinal fusion segment is divided into cervical fusion, thoraco-lumbar fusion, and others. Spinal non-fusion products, such as dynamic stabilization devices (or motion preservation devices) have become the treatment of choice (especially for young patients), in comparison to the other two segments of the spinal non-fusion segment, namely, interspinous process decompression (IPD) devices and total disc replacement.
Factors driving the demand for Spine Surgery Devices are the increasing incidence of spinal deformities such as disc compression amongst the aging population in the North American region, and the popularity of non-fusion devices such as artificial spinal disc and nucleus has triggered the market. The North America spine surgery devices market is witnessing a high growth due to technological improvements in the treatment of spinal disorders. The increasing demand from the aging population, along with the advancement in medical technology (minimally invasive techniques and biologics), is driving the demand for the spine surgery device market in this region.
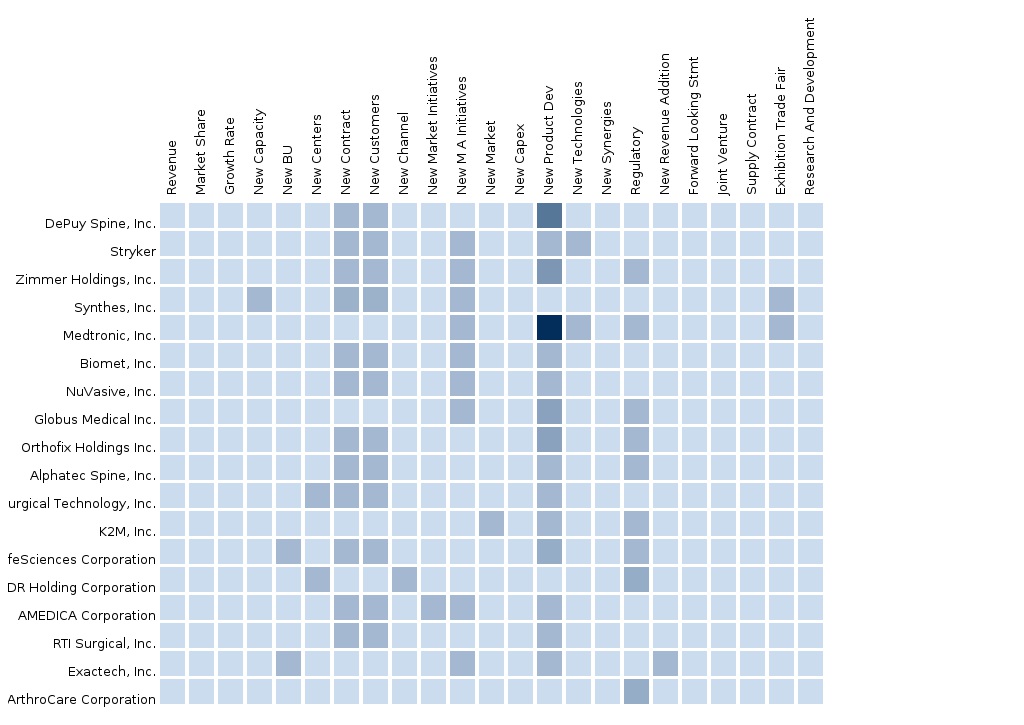
The North American Spine Surgery Devices market is estimated to be dominated by new product launches, & Mergers & Acquisitions are the major strategies adopted by most market players to achieve growth in the North American Spine Surgery Devices market. In November 2014, Zimmer Holdings Inc. (U.S.) launched Zimmer launched Virage® OCT Spinal Fixation System. This system is featured with the 360 degree Omnidirectional Extreme-Angle Screw, which allows a 112 degree conical range of motion. This system is designed to minimize the required operating time and to simplify rod alignment. Likewise, in October 2014, Medtronic Plc, one of the leading orthopedic medical technology companies launched the Pure Titanium Coating (PTC) platform of interbody fusion devices. This platform includes CAPSTONE PTC(TM) Spinal System, CLYDESDALE PTC(TM) Spinal System, ANATOMIC PEEK PTC Cervical Fusion System and CORNERSTONE-SR® Ti- Coated Anatomical Cervical Cage. Compression of the nerve roots or spinal cord causes pain, this device will help to reduce this pain by restoring the normal height of discs.
In-depth Market share analysis, by revenue, of the top companies is also included in the report. These numbers are arrived at, based on key facts, annual financial information from SEC filings, Annual reports and interviews with industry experts, key opinion leaders such as CEOs, directors, and marketing executives. Top market players that have established their base in the Europe spine surgery devices market are Stryker Corporation (U.S.), DePuy Synthes Companies of Johnson & Johnson (U.S.), Zimmer Inc (U.S.), Medtronic (U.S.), Alphatec Spine, Inc (U.S), NuVasive Inc (U.S.), Integra Lifesciences Holdings Corporation (U.S.), Orthofix International N.V. (U.S), Globus Medical Inc. (U.S), LDR Holding Corporation (U.S), K2M Inc. (U.S.), and B. Braun Melsungen (Germany), and other companies.
Reasons to Buy the Report:
From an insight perspective, this research report has focused on various levels of analysis—industry analysis (industry trends, and PEST analysis), market share analysis of top players, supply chain analysis, and company profiles, which together comprise and discuss the basic views on the competitive landscape, emerging- and high-growth segments of the North American Spine Surgery Devices market, high-growth regions and countries and their respective regulatory policies, government initiatives, drivers, restraints, and opportunities.
The report will enrich both established firms as well as new entrants/smaller firms to gauge the pulse of the market, which in turn will help the firms in garnering a greater market share. Firms purchasing the report could use any one or combination of the below mentioned five strategies (market penetration, product development/innovation, market development, market diversification, and competitive assessment) for strengthening their market share.
The report provides insights on the following pointers:
- Market Penetration: Comprehensive information on Spine Surgery Devices products offered by the top players in the spine surgery devices market
- Product Development/Innovation: Detailed insights on upcoming technologies, research and development activities, and new product launches in the spine surgery devices market
- Market Development: Comprehensive information about lucrative emerging markets.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the spine surgery devices market
- Competitive Assessment: In-depth assessment of market shares, strategies, products, and manufacturing capabilities of leading players in the spine surgery devices market
Tables of Contents
1 Introduction
1.1 Objectives of the Study
1.2 Market Segmentation & Coverage
1.3 Stakeholders
2 Research Methodology
2.1 Integrated Ecosystem of the Spine Surgery Devices Market
2.2 Arriving at the Spine Surgery Devices Market Size
2.2.1 Top-Down Approach
2.2.2 Demand-Side Approach
2.2.3 Macroindicator-Based Approach
2.3 Assumptions
3 Executive Summary
4 Market Overview
4.1 Introduction
4.2 Spine Surgery Devices Market: Comparison With Parent Market
4.3 Market Drivers And Inhibitors
4.4 Key Market Dynamics
5 North America Spine Surgery Devices Market, By Product
5.1 Introduction
5.2 North American Spine Surgery Devices Market, By Product: Comparison With Orthopedic Devices Market
5.3 Spinal Fusion & Fixation Devices
5.3.1 Cervical Fusion
5.3.2 Thoracic Fusion & Lumbar Fusion
5.3.2.1 Anterior Thoracic Fusion And Lumbar Fusion
5.3.2.2 Posterior Thoracolumbar Fusion
5.3.2.3 Interbody Thoracic Fusion And Lumbar Fusion
5.3.2.3.1 By Approach
5.3.2.3.1.1 Anterior Lumbar Interbody Fusion
5.3.2.3.1.2 Posterior Lumbar Interbody Fusion
5.3.2.3.1.3 Transforaminal Lumbar Interbody Fusion
5.3.2.3.1.4 Lateral Lumbar Interbody Fusion
5.3.2.3.2 By Material
5.3.2.3.2.1 Non-Bone Interbody Cervical Fusion Devices
5.3.2.3.2.2 Bone Interbody Fusion Devices
5.4 Spine Biologics
5.4.1 Spinal Allografts
5.4.2 Demineralized Bone Matrix
5.4.3 Bone Morphogenetic Proteins
5.4.4 Bone Substitutes
5.4.5 Machined Bones
5.5 Vertebral Compression Fracture (Vcf) Treatment Devices
5.5.1 Vertebroplasty
5.5.2 Balloon Kyphoplasty Devices
5.6 Spine Bone Stimulators
5.6.1 Electrical Stimulators
5.6.1.1 Noninvasive Bone Stimulators
5.6.1.1.1 Pulsed Electromagnetic Field Devices
5.6.1.1.2 Capacitive Coupling(Cc) And Combined (Electro) Magnetic Field (Cmf) Devices
5.6.1.2 Invasive Electrical Bone Stimulators
5.7 Non-Fusion Implants
5.7.1 Dynamic Stabilization
5.7.1.1 Interspinous Process Spacers
5.7.1.2 Pedicle-Screw Based Systems
5.7.1.3 Facet Replacement Products
5.7.2 Artificial Disc Replacement
5.7.2.1 Artificial Cervical Discs
5.7.2.2 Artificial Lumbar Discs
6 North America Spine Surgery Devices Market, By End User
6.1 Introduction
6.2 North America: Spine Surgery Devices Market in Hospitals, By Country
6.3 North America: Spine Surgery Devices Market in Orthopedic Clinics, By Country
7 North America Spine Surgery Devices Market, By Country
7.1 Introduction
7.2 U.S.: Spine Surgery Devices Market
7.2.1 U.S.: Spine Surgery Devices Market, By Product
7.2.2 U.S.: Spine Surgery Devices Market, By End User
7.3 Canada: Spine Surgery Devices Market
7.3.1 Canada: Spine Surgery Devices Market, By Product
7.3.2 Canada: Spine Surgery Devices Market, By End User
7.4 Mexico: Spine Surgery Devices Market
7.4.1 Mexico: Spine Surgery Devices Market, By Product
7.4.2 Mexico: Spine Surgery Devices Market, By End User
8 North America Spine Surgery Devices Market: Competitive Landscape
8.1 North America: Spine Surgery Devices Market: Company Share Analysis
8.2 Mergers & Acquisitions
8.3 New Product Launches
8.4 Agreements
8.5 Approvals
8.6 Investments
9 Spine Surgery Market, By Company
9.1 Alphatec Spine, Inc.
9.2 Biomet, Inc.
9.3 Depuy Synthes Companies.
9.4 Globus Medical, Inc.
9.5 Integra Lifesciences Holdings Corporation.
9.6 Medtronic, Inc.
9.7 Nuvasive, Inc.
9.8 Stryker Corporation
9.9 Zimmer Holding, Inc.
9.10 Amedica Corporation
10 Appendix
10.1 Customization Options
10.1.1 Product Analysis:
10.1.2 Epidemiology Data:
10.1.3 Procedure Volume Data:
10.1.4 Surgeons’/Physicians’ Perception Analysis:
10.1.5 Brand/Product Perception Matrix:
10.1.6 Regulatory Framework
10.1.7 Alternative Products: Impact Analysis
10.2 Related Reports
10.3 Introducing RT: Real-Time Market Intelligence
10.3.1 RT Snapshots
List of Tables (40 Tables)
Table 1 Global Spine Surgery Devices Peer Market Size, 2014 (Usd Mn)
Table 2 North America: Number of Road Accidents, 2014 (Absolute)
Table 3 North America: Aging Population, 2014 (Mn)
Table 4 North American Spine Surgery Devices Market: Macroindicators, By Country, 2014 (Usd Mn)
Table 5 North American Spine Surgery Devices Market: Comparison With Parent Market, 2013–2019 (Usd Mn)
Table 6 North American Spine Surgery Devices Market: Drivers And Inhibitors
Table 7 North America: Spine Surgery Devices Market, By Product, 2013 - 2019 (Usd Mn)
Table 8 North America: Spine Surgery Devices Market, By Geography, 2013 - 2019 (Usd Mn)
Table 9 North America: Spine Surgery Devices Market, By Product, 2013 - 2019 (Usd Mn)
Table 10 North American Spine Surgery Devices Market, By Product: Comparison With Orthopedic Devices Market, 2013–2019 (Usd Mn)
Table 11 North America: Spinal Fusion & Fixation Devices Market, By Country, 2013–2019 (Usd Mn)
Table 12 North America: Spine Biologics Market, By Country, 2013–2019 (Usd Mn)
Table 13 North America: Vcf Treatment Devices Market, By Country, 2013–2019 (Usd Mn)
Table 14 North America: Spine Bone Stimulators Market, By Country, 2013–2019 (Usd Mn)
Table 15 North America: Non-Fusion Implants Market, By Country, 2013–2019 (Usd Mn)
Table 16 North America: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Table 17 North America: Spine Surgery Devices Market in Hospitals, By Country, 2013-2019 (Usd Mn)
Table 18 North America: Spinal Surgery Devices Market in Orthopedic Clinics, By Country, 2013-2019 (Usd Mn)
Table 19 North American Spine Surgery Devices Market, By Country, 2013 - 2019 (Usd Mn)
Table 20 U.S.: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Table 21 U.S.: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Table 22 Canada: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Table 23 Canada: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Table 24 Mexico: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Table 25 Mexico: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Table 26 North America: Spine Surgery Devices Market: Company Share Analysis, 2013 (%)
Table 27 North America: Spine Surgery Devices Market: Mergers & Acquisitions
Table 28 North America: Spine Surgery Devices Market: New Product Launches
Table 29 North America: Spine Surgery Devices Market: Agreements
Table 30 North America Spine Surgery Devices Market: Approvals
Table 31 North America: Spine Surgery Devices Market: Investments
Table 32 Alphatec Spine, Inc.: Key Financials, 2012 - 2014 (Usd Mn)
Table 33 Biomet, Inc.: Key Financials, 2011 - 2013 (Usd Mn)
Table 34 Johnson & Johnson Ltd.: Key Financials, 2011 - 2014 (Usd Mn)
Table 35 Integra Lifesciences Holdings Corporation: Key Financials, 2011 - 2013 (Usd Mn)
Table 36 Medtronic, Inc. : Key Financials, By Segment, 2009 - 2013 (Usd Million)
Table 37 Nuvasive, Inc.: Key Financials, 2009 - 2013 (Usd Mn)
Table 38 Stryker Corporation.: Key Financials, 2011 - 2014 (Usd Mn)
Table 39 Zimmer Holding, Inc.: Key Financials, 2011 - 2013 (Usd Mn)
Table 40 Amedica Corporation.: Key Financials, 2011 - 2013 (Usd Mn)
List of Figures (42 Figures)
Figure 1 North America Spine Surgery Devices Market: Segmentation & Coverage
Figure 2 Spine Surgery Market: Integrated Ecosystem
Figure 3 Research Methodology
Figure 4 Top-Down Approach
Figure 5 Demand-Side Approach: Number of Road Accidents, 2014
Figure 6 Demand-Side Approach: Aging Population (Mn), 2014
Figure 7 Macroindicator-Based Approach, 2014
Figure 8 North America: Spine Surgery Devices Market Snapshot, 2014
Figure 9 North America Spine Surgery Devices Market: Comparison With Parent
Figure 10 North America: Spine Surgery Devices Products, By Country, 2014 (Usd Mn)
Figure 11 North America: Spine Surgery Devices Market, By Product, 2013 - 2019 (Usd Mn)
Figure 12 North American Spine Surgery Devices Market, By Product: Comparison With Orthopedic Devices Market, 2013–2019 (Usd Mn)
Figure 13 North America: Spinal Fusion & Fixation Devices Market, By Country, 2013–2019 (Usd Mn)
Figure 14 North America: Spine Biologics Market, By Country, 2013–2019 (Usd Mn)
Figure 15 North America: Vcf Treatment Devices Market, By Country, 2013–2019 (Usd Mn)
Figure 16 North America: Spine Bone Stimulators Market, By Country, 2013–2019 (Usd Mn)
Figure 17 North America: Non-Fusion Implants Market, By Country, 2013–2019 (Usd Mn)
Figure 18 North America: North America: Spine Surgery Devices Market in Hospitals, By Country, 2013-2019 (Usd Mn)
Figure 19 North America: Spine Surgery Devices Market in Orthopedic Clinics, By Country 2013-2019 (Usd Mn)
Figure 20 North American Spine Surgery Devices Market: Growth Analysis, By Country, 2013-2019 (Usd Mn)
Figure 21 U.S.: Spine Surgery Devices Market Overview, 2014 & 2019 (%)
Figure 22 U.S.: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Figure 23 U.S.: Spine Surgery Devices Market, By End User
Figure 24 U.S.: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Figure 25 Canada: Spine Surgery Devices Market Overview, 2014 & 2019 (%)
Figure 26 Canada: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Figure 27 Canada: Spine Surgery Devices Market, By End User
Figure 28 Canada: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Figure 29 Mexico: Spine Surgery Devices Market Overview, 2014 & 2019 (%)
Figure 30 Mexico: Spine Surgery Devices Market, By Product, 2013-2019 (Usd Mn)
Figure 31 Mexico: Spine Surgery Devices Market, By End User
Figure 32 Mexico: Spine Surgery Devices Market, By End User, 2013-2019 (Usd Mn)
Figure 33 North America: Spine Surgery Devices Market: Company Share Analysis, 2013 (%)
Figure 34 Alphatec Spine, Inc., : Revenue Mix 2014
Figure 35 Biomet, Inc.: Revenue Mix, 2013 (%)
Figure 36 Johnson & Johnson Ltd. Revenue Mix, 2014 (%)
Figure 37 Integra Lifesciences Holdings Corporation. Revenue Mix, 2013 (%)
Figure 38 Medtronic, Inc. Revenue Mix, 2014
Figure 39 Nuvasive, Inc.: Revenue Mix 2013 (%)
Figure 40 Stryker Corporation Revenue Mix, 2014 (%)
Figure 41 Zimmer Holding, Inc.: Revenue Mix, 2013 (%)
Figure 42 Amedica Corporation.: Revenue Mix, 2013 (%)
This report covers the definition, description, and forecast of the North American Spine Surgery devices market. It involves a detailed analysis of the market segmentation, based on product type, end user, and country. The report also provides a strategic analysis of the key players in this market. In terms of countries, the market is segmented into the U.S., Canada, and Mexico. This report also provides critical insights into emerging trends that will fuel product adoption and market growth for plates, screws, rod, wires & pins, fusion nails, and external fixation devices during the forecast period.
Trauma device manufacturers are becoming increasingly focused on developing products suited for specific anatomical locations. Low-profile plating systems, for example, have grown in popularity for extremity fixation procedures, where soft tissue coverage is minimal and thicker systems often cause irritation. Polyaxial plating systems are also penetrating the market because these systems improve screw placement, a particularly important feature when a surgeon is securing small bone fragments near periarticular surfaces. Market growth will also continue to be fueled by surgeon uptake of anatomic plating systems. These devices are designed to fit the unique contours of periarticular surfaces, allowing for improved screw placement around small bone fragments to achieve adequate fracture reduction. As surgeons are primarily focused on attaining the most secure fixation possible, significant market opportunities exist for companies that introduce advanced or specialized spine surgery technologies.
The North American spine surgery devices market is expected to reach $6,756.6 million by 2019, at a CAGR of 7.5% from 2014 to 2019. This is mainly due to the increasing incidence of spinal deformities such as disc compression among the elderly and the popularity of non-fusion devices such as artificial spinal discs and disc nuclei. The increasing demand for spine surgery devices from the aging population, along with advancements in medical technologies (minimally invasive techniques and biologics), is driving the demand for spinal surgery devices in this region.
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
Asia Orthopedics Device Market The report “Asian Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. The main companies operating in Asian Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
North America Orthopedic Devices The report “North American Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. Both the markets have been witnessing the maximum growth because of increase in patient pool and procedure numbers of hip and knee osteoarthritis and rheumatoid arthritis. The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in North American Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
Europe Orthopedics Device Market The report “European Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques.The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in European Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. |
Upcoming |












