Arthroscopy is a minimally invasive surgical procedure that is commonly used to evaluate the knees and shoulders, but can also be used to examine and treat conditions of the wrist, ankle, and elbow. An arthroscopy is performed using an instrument called the arthroscope, which is essentially an endoscope with a tube length that ranges from 110 mm to 187.5 mm. The tube has a camera attached and is inserted into a joint, through a small incision, to examine and treat damaged interior joints.
The North American arthroscopic devices report defines and segments the concerned market with an analysis and forecast of the revenue. The arthroscopic devices market in North America is estimated to grow to about $3963.9 million by 2018, at a CAGR of 6.9% from 2013 to 2018.
The rising awareness in patients about the benefits of minimally invasive procedures, such as reduced hospitalization and less traumatic surgical experience, has significantly increased the demand for the use of arthroscopic devices in North America (the U.S., Canada, and Mexico). These minimally invasive procedures are rapidly replacing open surgeries as a result of technological innovations. Developments in visualization and monitoring equipment such as arthroscopic devices for instance, have facilitated greater precision in joint and spinal surgeries by providing enhanced image quality. For instance, the development of arthroscopes has not only lessened the size of surgical incisions required in a typical joint replacement or spinal fusion surgery, but has also dramatically reduced the post-operative recovery time and hospital stays.
A rapid increase in the aging population in North America holds high potential for the growth of this market. An increasing use of these devices in joint replacement and spinal surgeries across North America has helped this segment in North America maintain the top position, globally. Moreover, an increase in obesity rate has been driving the orthopedic procedure growth.
This market is segmented and forecasted on the basis of six submarkets, namely Arthroscopes, Arthroscopic Hand Instruments, Drill Guide, Fluid Management Devices, Power Saver Systems, and Radiofrequency Probe. The market is further segmented and forecasted on the basis of major countries such as the U.S., Canada, and Mexico and on the basis of end-users.
Some of the arthroscopes available in the market are Karl Storz 7210 CA ( Karl Storz GmbH & Co.), PENTEX ES 3870K SCOPE (Pentax Medical Company), and Olympus Arthroscope 2.7mm (Olympus Medical System Corporation).
This report also includes the market share, value chain analysis, and market metrics such as drivers, restraints and upcoming opportunities in the market. In addition, it presents a competitive landscape and company profiles of key players in the market.
1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Arthroscopic Devices-North America, By Segments
4.1 Split By Geography
4.1 Arthroscopic Devices-U.S. by Markets
4.1 Arthroscopic Devices-Canada by Markets
4.1 Arthroscopic Devices-Mexico by Markets
4.2 Arthroscopes-North America
4.2.1 Arthroscopes-North America, By Endusers
4.2.1.1 Arthroscopes-North America-Hospitals
4.2.1.2 Arthroscopes-North America-Point of Care Testing
4.2.1.3 Arthroscopes-North America-Other Endusers
4.2.2 Arthroscopes-North America, By Geographies
4.2.2.1 Arthroscopes-U.S.
4.2.2.2 Arthroscopes-Canada
4.2.2.3 Arthroscopes-Mexico
4.3 Arthroscopic Hand Instruments-North America
4.3.1 Arthroscopic Hand Instruments-North America, By Endusers
4.3.1.1 Arthroscopic Hand Instruments-North America-Hospitals
4.3.1.2 Arthroscopic Hand Instruments-North America-Point of Care Testing
4.3.1.3 Arthroscopic Hand Instruments-North America-Other Endusers
4.3.2 Arthroscopic Hand Instruments-North America, By Geographies
4.3.2.1 Arthroscopic Hand Instruments-U.S.
4.3.2.2 Arthroscopic Hand Instruments-Canada
4.3.2.3 Arthroscopic Hand Instruments-Mexico
4.4 Fluid Management Devices-North America
4.4.1 Fluid Management Devices-North America, By Endusers
4.4.1.1 Fluid Management Devices-North America-Hospitals
4.4.1.2 Fluid Management Devices-North America-Point of Care Testing
4.4.1.3 Fluid Management Devices-North America-Other Endusers
4.4.2 Fluid Management Devices-North America, By Geographies
4.4.2.1 Fluid Management Devices-U.S.
4.4.2.2 Fluid Management Devices-Canada
4.4.2.3 Fluid Management Devices-Mexico
4.5 Power Saver Systems-North America
4.5.1 Power Saver Systems-North America, By Endusers
4.5.1.1 Power Saver Systems-North America-Hospitals
4.5.1.2 Power Saver Systems-North America-Point of Care Testing
4.5.1.3 Power Saver Systems-North America-Other Endusers
4.5.2 Power Saver Systems-North America, By Geographies
4.5.2.1 Power Saver Systems-U.S.
4.5.2.2 Power Saver Systems-Canada
4.5.2.3 Power Saver Systems-Mexico
4.6 Radiofrequency Probe-North America
4.6.1 Radiofrequency Probe-North America, By Endusers
4.6.1.1 Radiofrequency Probe-North America-Hospitals
4.6.1.2 Radiofrequency Probe-North America-Point of Care Testing
4.6.1.3 Radiofrequency Probe-North America-Other Endusers
4.6.2 Radiofrequency Probe-North America, By Geographies
4.6.2.1 Radiofrequency Probe-U.S.
4.6.2.2 Radiofrequency Probe-Canada
4.6.2.3 Radiofrequency Probe-Mexico
4.7 Drill Guide-North America
4.7.1 Drill Guide-North America, By Endusers
4.7.1.1 Drill Guide-North America-Hospitals
4.7.1.2 Drill Guide-North America-Point of Care Testing
4.7.1.3 Drill Guide-North America-Other Endusers
4.7.2 Drill Guide-North America, By Geographies
4.7.2.1 Drill Guide-U.S.
4.7.2.2 Drill Guide-Canada
4.7.2.3 Drill Guide-Mexico
5 Arthroscopic Devices-North America, By Endusers
5.1 Split By Geography
5.2 Arthroscopic Devices-U.S. by Endusers
5.1 Arthroscopic Devices-Canada by Endusers
5.1 Arthroscopic Devices-Mexico by Endusers
5.2 Arthroscopic Devices-North America-Hospitals
5.2.1 Arthroscopic Devices-North America-Hospitals, By Geographies
5.2.1.1 Arthroscopic Devices-U.S.-Hospitals
5.2.1.2 Arthroscopic Devices-Canada-Hospitals
5.2.1.3 Arthroscopic Devices-Mexico-Hospitals
5.2.2 Arthroscopic Devices-North America-Hospitals, By Segments
5.2.2.1 Arthroscopes-North America-Hospitals
5.2.2.2 Arthroscopic Hand Instruments-North America-Hospitals
5.2.2.3 Fluid Management Devices-North America-Hospitals
5.2.2.4 Power Saver Systems-North America-Hospitals
5.2.2.5 Radiofrequency Probe-North America-Hospitals
5.2.2.6 Drill Guide-North America-Hospitals
5.3 Arthroscopic Devices-North America-Point of Care Testing
5.3.1 Arthroscopic Devices-North America-Point of Care Testing, By Geographies
5.3.1.1 Arthroscopic Devices-U.S.-Point of Care Testing
5.3.1.2 Arthroscopic Devices-Canada-Point of Care Testing
5.3.1.3 Arthroscopic Devices-Mexico-Point of Care Testing
5.3.2 Arthroscopic Devices-North America-Point of Care Testing, By Segments
5.3.2.1 Arthroscopes-North America-Point of Care Testing
5.3.2.2 Arthroscopic Hand Instruments-North America-Point of Care Testing
5.3.2.3 Fluid Management Devices-North America-Point of Care Testing
5.3.2.4 Power Saver Systems-North America-Point of Care Testing
5.3.2.5 Radiofrequency Probe-North America-Point of Care Testing
5.3.2.6 Drill Guide-North America-Point of Care Testing
5.4 Arthroscopic Devices-North America-Other Endusers
5.4.1 Arthroscopic Devices-North America-Other Endusers, By Geographies
5.4.1.1 Arthroscopic Devices-U.S.-Other Endusers
5.4.1.2 Arthroscopic Devices-Canada-Other Endusers
5.4.1.3 Arthroscopic Devices-Mexico-Other Endusers
5.4.2 Arthroscopic Devices-North America-Other Endusers, By Segments
5.4.2.1 Arthroscopes-North America-Other Endusers
5.4.2.2 Arthroscopic Hand Instruments-North America-Other Endusers
5.4.2.3 Fluid Management Devices-North America-Other Endusers
5.4.2.4 Power Saver Systems-North America-Other Endusers
5.4.2.5 Radiofrequency Probe-North America-Other Endusers
5.4.2.6 Drill Guide-North America-Other Endusers
6 Arthroscopic Devices-North America, By Geographies
6.1 Arthroscopic Devices-U.S.
6.1.1 Arthroscopic Devices-U.S., By Endusers
6.1.1.1 Arthroscopic Devices-U.S.-Hospitals
6.1.1.2 Arthroscopic Devices-U.S.-Point of Care Testing
6.1.1.3 Arthroscopic Devices-U.S.-Other Endusers
6.1.2 Arthroscopic Devices-U.S., By Segments
6.1.2.1 Arthroscopes-U.S.
6.1.2.2 Arthroscopic Hand Instruments-U.S.
6.1.2.3 Fluid Management Devices-U.S.
6.1.2.4 Power Saver Systems-U.S.
6.1.2.5 Radiofrequency Probe-U.S.
6.1.2.6 Drill Guide-U.S.
6.2 Arthroscopic Devices-Canada
6.2.1 Arthroscopic Devices-Canada, By Endusers
6.2.1.1 Arthroscopic Devices-Canada-Hospitals
6.2.1.2 Arthroscopic Devices-Canada-Point of Care Testing
6.2.1.3 Arthroscopic Devices-Canada-Other Endusers
6.2.2 Arthroscopic Devices-Canada, By Segments
6.2.2.1 Arthroscopes-Canada
6.2.2.2 Arthroscopic Hand Instruments-Canada
6.2.2.3 Fluid Management Devices-Canada
6.2.2.4 Power Saver Systems-Canada
6.2.2.5 Radiofrequency Probe-Canada
6.2.2.6 Drill Guide-Canada
6.3 Arthroscopic Devices-Mexico
6.3.1 Arthroscopic Devices-Mexico, By Endusers
6.3.1.1 Arthroscopic Devices-Mexico-Hospitals
6.3.1.2 Arthroscopic Devices-Mexico-Point of Care Testing
6.3.1.3 Arthroscopic Devices-Mexico-Other Endusers
6.3.2 Arthroscopic Devices-Mexico, By Segments
6.3.2.1 Arthroscopes-Mexico
6.3.2.2 Arthroscopic Hand Instruments-Mexico
6.3.2.3 Fluid Management Devices-Mexico
6.3.2.4 Power Saver Systems-Mexico
6.3.2.5 Radiofrequency Probe-Mexico
6.3.2.6 Drill Guide-Mexico
7 Arthroscopic Devices-North America, By Companies
7.1 Competitive landscape
7.2 Split By Geography
7.3 Arthroscopic Devices-U.S. by Companies
7.1 Arthroscopic Devices-Canada by Companies
7.1 Arthroscopic Devices-Mexico by Companies
7.3 Arthroscopic Devices-North America-Other Companies
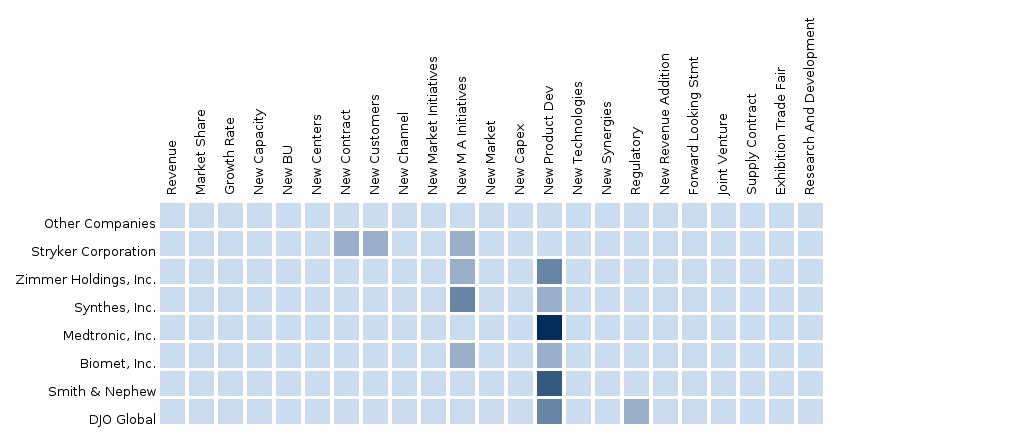
7.4 Arthroscopic Devices-North America-Stryker Corporation
7.5 Arthroscopic Devices-North America-Zimmer Holdings, Inc.
7.6 Arthroscopic Devices-North America-Synthes, Inc.
7.7 Arthroscopic Devices-North America-Medtronic, Inc.
7.8 Arthroscopic Devices-North America-Biomet, Inc.
7.9 Arthroscopic Devices-North America-Smith & Nephew
7.10 Arthroscopic Devices-North America-DJO Global
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
Asia Orthopedics Device Market The report “Asian Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. The main companies operating in Asian Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
North America Orthopedic Devices The report “North American Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. Both the markets have been witnessing the maximum growth because of increase in patient pool and procedure numbers of hip and knee osteoarthritis and rheumatoid arthritis. The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in North American Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
Europe Orthopedics Device Market The report “European Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques.The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in European Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. |
Upcoming |












