1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Clinical Trial Management Systems (CTMS)-Europe, By Deployments
4.1 Split By Geography
4.1 Clinical Trial Management Systems (CTMS)-Spain by Deployments
4.1 Clinical Trial Management Systems (CTMS)-United Kingdom by Deployments
4.1 Clinical Trial Management Systems (CTMS)-France by Deployments
4.1 Clinical Trial Management Systems (CTMS)-Germany by Deployments
4.1 Clinical Trial Management Systems (CTMS)-Italy by Deployments
4.2 Clinical Trial Management Systems (CTMS)-Web-based-Europe
4.2.1 Clinical Trial Management Systems (CTMS)-Web-based-Europe, By Endusers
4.2.1.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-Web-based
4.2.1.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-Web-based
4.2.1.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-Web-based
4.2.2 Clinical Trial Management Systems (CTMS)-Web-based-Europe, By Geographies
4.2.2.1 Clinical Trial Management Systems (CTMS)-Spain-Web-based
4.2.2.2 Clinical Trial Management Systems (CTMS)-United Kingdom-Web-based
4.2.2.3 Clinical Trial Management Systems (CTMS)-France-Web-based
4.2.2.4 Clinical Trial Management Systems (CTMS)-Germany-Web-based
4.2.2.5 Clinical Trial Management Systems (CTMS)-Italy-Web-based
4.3 Clinical Trial Management Systems (CTMS)-On-premise-Europe
4.3.1 Clinical Trial Management Systems (CTMS)-On-premise-Europe, By Endusers
4.3.1.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-On-premise
4.3.1.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-On-premise
4.3.1.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-On-premise
4.3.2 Clinical Trial Management Systems (CTMS)-On-premise-Europe, By Geographies
4.3.2.1 Clinical Trial Management Systems (CTMS)-Spain-On-premise
4.3.2.2 Clinical Trial Management Systems (CTMS)-United Kingdom-On-premise
4.3.2.3 Clinical Trial Management Systems (CTMS)-France-On-premise
4.3.2.4 Clinical Trial Management Systems (CTMS)-Germany-On-premise
4.3.2.5 Clinical Trial Management Systems (CTMS)-Italy-On-premise
4.4 Clinical Trial Management Systems (CTMS)-Cloud-based-Europe
4.4.1 Clinical Trial Management Systems (CTMS)-Cloud-based-Europe, By Endusers
4.4.1.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-Cloud-based
4.4.1.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-Cloud-based
4.4.1.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-Cloud-based
4.4.2 Clinical Trial Management Systems (CTMS)-Cloud-based-Europe, By Geographies
4.4.2.1 Clinical Trial Management Systems (CTMS)-Spain-Cloud-based
4.4.2.2 Clinical Trial Management Systems (CTMS)-United Kingdom-Cloud-based
4.4.2.3 Clinical Trial Management Systems (CTMS)-France-Cloud-based
4.4.2.4 Clinical Trial Management Systems (CTMS)-Germany-Cloud-based
4.4.2.5 Clinical Trial Management Systems (CTMS)-Italy-Cloud-based
5 Clinical Trial Management Systems (CTMS)-Europe, By Components
5.1 Split By Geography
5.2 Clinical Trial Management Systems (CTMS)-Spain by Components
5.1 Clinical Trial Management Systems (CTMS)-United Kingdom by Components
5.1 Clinical Trial Management Systems (CTMS)-France by Components
5.1 Clinical Trial Management Systems (CTMS)-Germany by Components
5.1 Clinical Trial Management Systems (CTMS)-Italy by Components
5.2 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Europe
5.2.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Europe, By Endusers
5.2.1.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Healthcare Providers-Europe
5.2.1.2 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Clinical Research Organization-Europe
5.2.1.3 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Pharmaceuticals-Europe
5.2.2 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Europe, By Geographies
5.2.2.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Spain
5.2.2.2 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-United Kingdom
5.2.2.3 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-France
5.2.2.4 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Germany
5.2.2.5 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Italy
5.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Europe
5.3.1 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Europe, By Endusers
5.3.1.1 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Healthcare Providers-Europe
5.3.1.2 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Clinical Research Organization-Europe
5.3.1.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Pharmaceuticals-Europe
5.3.2 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Europe, By Geographies
5.3.2.1 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Spain
5.3.2.2 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-United Kingdom
5.3.2.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-France
5.3.2.4 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Germany
5.3.2.5 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Italy
5.4 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Europe
5.4.1 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Europe, By Endusers
5.4.1.1 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Healthcare Providers-Europe
5.4.1.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Clinical Research Organization-Europe
5.4.1.3 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Pharmaceuticals-Europe
5.4.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Europe, By Geographies
5.4.2.1 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Spain
5.4.2.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-United Kingdom
5.4.2.3 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-France
5.4.2.4 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Germany
5.4.2.5 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Italy
6 Clinical Trial Management Systems (CTMS)-Europe, By Endusers
6.1 Split By Geography
6.3 Clinical Trial Management Systems (CTMS)-Spain by Endusers
6.1 Clinical Trial Management Systems (CTMS)-United Kingdom by Endusers
6.1 Clinical Trial Management Systems (CTMS)-France by Endusers
6.1 Clinical Trial Management Systems (CTMS)-Germany by Endusers
6.1 Clinical Trial Management Systems (CTMS)-Italy by Endusers
6.2 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe
6.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe, By Components
6.2.1.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Healthcare Providers-Europe
6.2.1.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Healthcare Providers-Europe
6.2.1.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Healthcare Providers-Europe
6.2.2 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe, By Deployments
6.2.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-Web-based
6.2.2.2 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-On-premise
6.2.2.3 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe-Cloud-based
6.2.3 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe, By Geographies
6.2.3.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Spain
6.2.3.2 Clinical Trial Management Systems (CTMS)-Healthcare Providers-United Kingdom
6.2.3.3 Clinical Trial Management Systems (CTMS)-Healthcare Providers-France
6.2.3.4 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Germany
6.2.3.5 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Italy
6.2.4 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Europe, By Types
6.2.4.1 Site CTMS-Healthcare Providers-Europe
6.2.4.2 Enterprise CTMS-Healthcare Providers-Europe
6.3 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe
6.3.1 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe, By Components
6.3.1.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Clinical Research Organization-Europe
6.3.1.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Clinical Research Organization-Europe
6.3.1.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Clinical Research Organization-Europe
6.3.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe, By Deployments
6.3.2.1 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-Web-based
6.3.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-On-premise
6.3.2.3 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe-Cloud-based
6.3.3 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe, By Geographies
6.3.3.1 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Spain
6.3.3.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-United Kingdom
6.3.3.3 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-France
6.3.3.4 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Germany
6.3.3.5 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Italy
6.3.4 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe, By Types
6.3.4.1 Site CTMS-Clinical Research Organization-Europe
6.3.4.2 Enterprise CTMS-Clinical Research Organization-Europe
6.4 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe
6.4.1 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe, By Components
6.4.1.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Pharmaceuticals-Europe
6.4.1.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Pharmaceuticals-Europe
6.4.1.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Pharmaceuticals-Europe
6.4.2 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe, By Deployments
6.4.2.1 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-Web-based
6.4.2.2 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-On-premise
6.4.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe-Cloud-based
6.4.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe, By Endusers
6.4.3.1 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Europe
6.4.4 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe, By Geographies
6.4.4.1 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Spain
6.4.4.2 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-United Kingdom
6.4.4.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-France
6.4.4.4 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Germany
6.4.4.5 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Italy
6.4.5 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Europe, By Types
6.4.5.1 Site CTMS-Pharmaceuticals-Europe
6.4.5.2 Enterprise CTMS-Pharmaceuticals-Europe
7 Clinical Trial Management Systems (CTMS)-Europe, By Types
7.1 Split By Geography
7.4 Clinical Trial Management Systems (CTMS)-Spain by Types
7.1 Clinical Trial Management Systems (CTMS)-United Kingdom by Types
7.1 Clinical Trial Management Systems (CTMS)-France by Types
7.1 Clinical Trial Management Systems (CTMS)-Germany by Types
7.1 Clinical Trial Management Systems (CTMS)-Italy by Types
7.2 Enterprise CTMS-Europe
7.2.1 Enterprise CTMS-Europe, By Endusers
7.2.1.1 Enterprise CTMS-Healthcare Providers-Europe
7.2.1.2 Enterprise CTMS-Clinical Research Organization-Europe
7.2.1.3 Enterprise CTMS-Pharmaceuticals-Europe
7.2.2 Enterprise CTMS-Europe, By Geographies
7.2.2.1 Enterprise CTMS-Spain
7.2.2.2 Enterprise CTMS-United Kingdom
7.2.2.3 Enterprise CTMS-France
7.2.2.4 Enterprise CTMS-Germany
7.2.2.5 Enterprise CTMS-Italy
7.3 Site CTMS-Europe
7.3.1 Site CTMS-Europe, By Endusers
7.3.1.1 Site CTMS-Healthcare Providers-Europe
7.3.1.2 Site CTMS-Clinical Research Organization-Europe
7.3.1.3 Site CTMS-Pharmaceuticals-Europe
7.3.2 Site CTMS-Europe, By Geographies
7.3.2.1 Site CTMS-Spain
7.3.2.2 Site CTMS-United Kingdom
7.3.2.3 Site CTMS-France
7.3.2.4 Site CTMS-Germany
7.3.2.5 Site CTMS-Italy
8 Clinical Trial Management Systems (CTMS)-Europe, By Geographies
8.1 Clinical Trial Management Systems (CTMS)-Spain
8.1.1 Clinical Trial Management Systems (CTMS)-Spain, By Companies
8.1.1.1 Clinical Trial Management Systems (CTMS)-Spain-Oracle Corporation
8.1.1.2 Clinical Trial Management Systems (CTMS)-Spain-PAREXEL International Corporation
8.1.1.3 Clinical Trial Management Systems (CTMS)-Spain-eResearch Technology Inc.
8.1.1.4 Clinical Trial Management Systems (CTMS)-Spain-Bioclinica.
8.1.1.5 Clinical Trial Management Systems (CTMS)-Spain-Datatrak international Inc.
8.1.1.6 Clinical Trial Management Systems (CTMS)-Spain-Pharmaceutical Product Development, LLC
8.1.2 Clinical Trial Management Systems (CTMS)-Spain, By Endusers
8.1.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Spain
8.1.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Spain
8.1.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Spain
8.1.3 Clinical Trial Management Systems (CTMS)-Spain, By Components
8.1.3.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Spain
8.1.3.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Spain
8.1.3.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Spain
8.1.4 Clinical Trial Management Systems (CTMS)-Spain, By Deployments
8.1.4.1 Clinical Trial Management Systems (CTMS)-Spain-Web-based
8.1.4.2 Clinical Trial Management Systems (CTMS)-Spain-On-premise
8.1.4.3 Clinical Trial Management Systems (CTMS)-Spain-Cloud-based
8.1.5 Clinical Trial Management Systems (CTMS)-Spain, By Types
8.1.5.1 Site CTMS-Spain
8.1.5.2 Enterprise CTMS-Spain
8.2 Clinical Trial Management Systems (CTMS)-United Kingdom
8.2.1 Clinical Trial Management Systems (CTMS)-United Kingdom, By Companies
8.2.1.1 Clinical Trial Management Systems (CTMS)-United Kingdom-Oracle Corporation
8.2.1.2 Clinical Trial Management Systems (CTMS)-United Kingdom-PAREXEL International Corporation
8.2.1.3 Clinical Trial Management Systems (CTMS)-United Kingdom-eResearch Technology Inc.
8.2.1.4 Clinical Trial Management Systems (CTMS)-United Kingdom-Bioclinica.
8.2.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-Datatrak international Inc.
8.2.1.6 Clinical Trial Management Systems (CTMS)-United Kingdom-Pharmaceutical Product Development, LLC
8.2.2 Clinical Trial Management Systems (CTMS)-United Kingdom, By Endusers
8.2.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-United Kingdom
8.2.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-United Kingdom
8.2.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-United Kingdom
8.2.3 Clinical Trial Management Systems (CTMS)-United Kingdom, By Components
8.2.3.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-United Kingdom
8.2.3.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-United Kingdom
8.2.3.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-United Kingdom
8.2.4 Clinical Trial Management Systems (CTMS)-United Kingdom, By Deployments
8.2.4.1 Clinical Trial Management Systems (CTMS)-United Kingdom-Web-based
8.2.4.2 Clinical Trial Management Systems (CTMS)-United Kingdom-On-premise
8.2.4.3 Clinical Trial Management Systems (CTMS)-United Kingdom-Cloud-based
8.2.5 Clinical Trial Management Systems (CTMS)-United Kingdom, By Types
8.2.5.1 Site CTMS-United Kingdom
8.2.5.2 Enterprise CTMS-United Kingdom
8.3 Clinical Trial Management Systems (CTMS)-France
8.3.1 Clinical Trial Management Systems (CTMS)-France, By Companies
8.3.1.1 Clinical Trial Management Systems (CTMS)-France-Oracle Corporation
8.3.1.2 Clinical Trial Management Systems (CTMS)-France-PAREXEL International Corporation
8.3.1.3 Clinical Trial Management Systems (CTMS)-France-eResearch Technology Inc.
8.3.1.4 Clinical Trial Management Systems (CTMS)-France-Bioclinica.
8.3.1.5 Clinical Trial Management Systems (CTMS)-France-Datatrak international Inc.
8.3.1.6 Clinical Trial Management Systems (CTMS)-France-Pharmaceutical Product Development, LLC
8.3.2 Clinical Trial Management Systems (CTMS)-France, By Endusers
8.3.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-France
8.3.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-France
8.3.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-France
8.3.3 Clinical Trial Management Systems (CTMS)-France, By Components
8.3.3.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-France
8.3.3.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-France
8.3.3.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-France
8.3.4 Clinical Trial Management Systems (CTMS)-France, By Deployments
8.3.4.1 Clinical Trial Management Systems (CTMS)-France-Web-based
8.3.4.2 Clinical Trial Management Systems (CTMS)-France-On-premise
8.3.4.3 Clinical Trial Management Systems (CTMS)-France-Cloud-based
8.3.5 Clinical Trial Management Systems (CTMS)-France, By Types
8.3.5.1 Site CTMS-France
8.3.5.2 Enterprise CTMS-France
8.4 Clinical Trial Management Systems (CTMS)-Germany
8.4.1 Clinical Trial Management Systems (CTMS)-Germany, By Companies
8.4.1.1 Clinical Trial Management Systems (CTMS)-Germany-Oracle Corporation
8.4.1.2 Clinical Trial Management Systems (CTMS)-Germany-PAREXEL International Corporation
8.4.1.3 Clinical Trial Management Systems (CTMS)-Germany-eResearch Technology Inc.
8.4.1.4 Clinical Trial Management Systems (CTMS)-Germany-Bioclinica.
8.4.1.5 Clinical Trial Management Systems (CTMS)-Germany-Datatrak international Inc.
8.4.1.6 Clinical Trial Management Systems (CTMS)-Germany-Pharmaceutical Product Development, LLC
8.4.2 Clinical Trial Management Systems (CTMS)-Germany, By Endusers
8.4.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Germany
8.4.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Germany
8.4.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Germany
8.4.3 Clinical Trial Management Systems (CTMS)-Germany, By Components
8.4.3.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Germany
8.4.3.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Germany
8.4.3.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Germany
8.4.4 Clinical Trial Management Systems (CTMS)-Germany, By Deployments
8.4.4.1 Clinical Trial Management Systems (CTMS)-Germany-Web-based
8.4.4.2 Clinical Trial Management Systems (CTMS)-Germany-On-premise
8.4.4.3 Clinical Trial Management Systems (CTMS)-Germany-Cloud-based
8.4.5 Clinical Trial Management Systems (CTMS)-Germany, By Types
8.4.5.1 Site CTMS-Germany
8.4.5.2 Enterprise CTMS-Germany
8.5 Clinical Trial Management Systems (CTMS)-Italy
8.5.1 Clinical Trial Management Systems (CTMS)-Italy, By Companies
8.5.1.1 Clinical Trial Management Systems (CTMS)-Italy-Oracle Corporation
8.5.1.2 Clinical Trial Management Systems (CTMS)-Italy-PAREXEL International Corporation
8.5.1.3 Clinical Trial Management Systems (CTMS)-Italy-eResearch Technology Inc.
8.5.1.4 Clinical Trial Management Systems (CTMS)-Italy-Bioclinica.
8.5.1.5 Clinical Trial Management Systems (CTMS)-Italy-Datatrak international Inc.
8.5.1.6 Clinical Trial Management Systems (CTMS)-Italy-Pharmaceutical Product Development, LLC
8.5.2 Clinical Trial Management Systems (CTMS)-Italy, By Endusers
8.5.2.1 Clinical Trial Management Systems (CTMS)-Healthcare Providers-Italy
8.5.2.2 Clinical Trial Management Systems (CTMS)-Clinical Research Organization-Italy
8.5.2.3 Clinical Trial Management Systems (CTMS)-Pharmaceuticals-Italy
8.5.3 Clinical Trial Management Systems (CTMS)-Italy, By Components
8.5.3.1 Clinical Trial Management Systems (CTMS)-Software (Healthcare IT)-Italy
8.5.3.2 Clinical Trial Management Systems (CTMS)-Hardware (Healthcare IT)-Italy
8.5.3.3 Clinical Trial Management Systems (CTMS)-Services (Healthcare IT)-Italy
8.5.4 Clinical Trial Management Systems (CTMS)-Italy, By Deployments
8.5.4.1 Clinical Trial Management Systems (CTMS)-Italy-Web-based
8.5.4.2 Clinical Trial Management Systems (CTMS)-Italy-On-premise
8.5.4.3 Clinical Trial Management Systems (CTMS)-Italy-Cloud-based
8.5.5 Clinical Trial Management Systems (CTMS)-Italy, By Types
8.5.5.1 Site CTMS-Italy
8.5.5.2 Enterprise CTMS-Italy
9 Clinical Trial Management Systems (CTMS)-Europe, By Companies
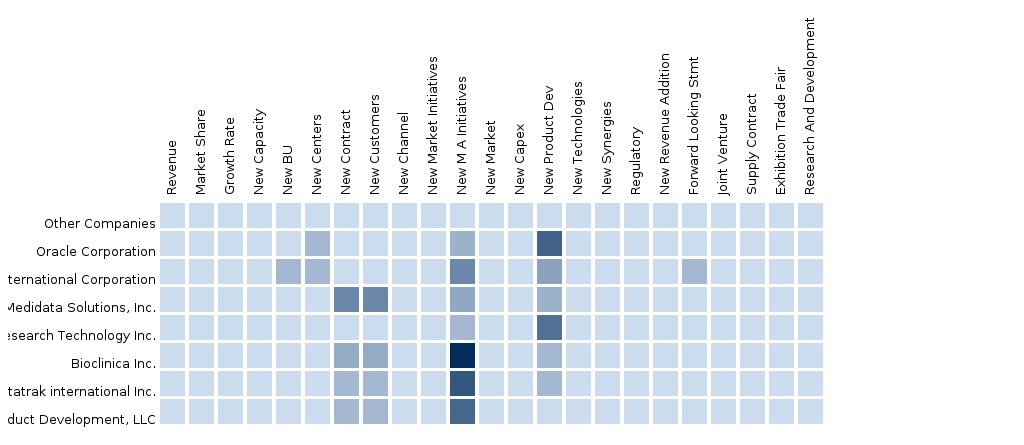
9.1 Competitive landscape
9.2 Split By Geography
9.5 Clinical Trial Management Systems (CTMS)-Spain by Companies
9.1 Clinical Trial Management Systems (CTMS)-United Kingdom by Companies
9.1 Clinical Trial Management Systems (CTMS)-France by Companies
9.1 Clinical Trial Management Systems (CTMS)-Germany by Companies
9.1 Clinical Trial Management Systems (CTMS)-Italy by Companies
9.3 Clinical Trial Management Systems (CTMS)-Europe-Oracle Corporation
9.3.1 Clinical Trial Management Systems (CTMS)-Europe-Oracle Corporation, By Geographies
9.3.1.1 Clinical Trial Management Systems (CTMS)-France-Oracle Corporation
9.3.1.2 Clinical Trial Management Systems (CTMS)-Germany-Oracle Corporation
9.3.1.3 Clinical Trial Management Systems (CTMS)-Italy-Oracle Corporation
9.3.1.4 Clinical Trial Management Systems (CTMS)-Spain-Oracle Corporation
9.3.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-Oracle Corporation
9.4 Clinical Trial Management Systems (CTMS)-Europe-PAREXEL International Corporation
9.4.1 Clinical Trial Management Systems (CTMS)-Europe-PAREXEL International Corporation, By Geographies
9.4.1.1 Clinical Trial Management Systems (CTMS)-France-PAREXEL International Corporation
9.4.1.2 Clinical Trial Management Systems (CTMS)-Germany-PAREXEL International Corporation
9.4.1.3 Clinical Trial Management Systems (CTMS)-Italy-PAREXEL International Corporation
9.4.1.4 Clinical Trial Management Systems (CTMS)-Spain-PAREXEL International Corporation
9.4.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-PAREXEL International Corporation
9.5 Clinical Trial Management Systems (CTMS)-Europe-eResearch Technology Inc.
9.5.1 Clinical Trial Management Systems (CTMS)-Europe-eResearch Technology Inc., By Geographies
9.5.1.1 Clinical Trial Management Systems (CTMS)-France-eResearch Technology Inc.
9.5.1.2 Clinical Trial Management Systems (CTMS)-Germany-eResearch Technology Inc.
9.5.1.3 Clinical Trial Management Systems (CTMS)-Italy-eResearch Technology Inc.
9.5.1.4 Clinical Trial Management Systems (CTMS)-Spain-eResearch Technology Inc.
9.5.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-eResearch Technology Inc.
9.6 Clinical Trial Management Systems (CTMS)-Europe-Bioclinica.
9.6.1 Clinical Trial Management Systems (CTMS)-Europe-Bioclinica., By Geographies
9.6.1.1 Clinical Trial Management Systems (CTMS)-France-Bioclinica.
9.6.1.2 Clinical Trial Management Systems (CTMS)-Germany-Bioclinica.
9.6.1.3 Clinical Trial Management Systems (CTMS)-Italy-Bioclinica.
9.6.1.4 Clinical Trial Management Systems (CTMS)-Spain-Bioclinica.
9.6.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-Bioclinica.
9.7 Clinical Trial Management Systems (CTMS)-Europe-Datatrak international Inc.
9.7.1 Clinical Trial Management Systems (CTMS)-Europe-Datatrak international Inc., By Geographies
9.7.1.1 Clinical Trial Management Systems (CTMS)-France-Datatrak international Inc.
9.7.1.2 Clinical Trial Management Systems (CTMS)-Germany-Datatrak international Inc.
9.7.1.3 Clinical Trial Management Systems (CTMS)-Italy-Datatrak international Inc.
9.7.1.4 Clinical Trial Management Systems (CTMS)-Spain-Datatrak international Inc.
9.7.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-Datatrak international Inc.
9.8 Clinical Trial Management Systems (CTMS)-Europe-Pharmaceutical Product Development, LLC
9.8.1 Clinical Trial Management Systems (CTMS)-Europe-Pharmaceutical Product Development, LLC, By Geographies
9.8.1.1 Clinical Trial Management Systems (CTMS)-France-Pharmaceutical Product Development, LLC
9.8.1.2 Clinical Trial Management Systems (CTMS)-Germany-Pharmaceutical Product Development, LLC
9.8.1.3 Clinical Trial Management Systems (CTMS)-Italy-Pharmaceutical Product Development, LLC
9.8.1.4 Clinical Trial Management Systems (CTMS)-Spain-Pharmaceutical Product Development, LLC
9.8.1.5 Clinical Trial Management Systems (CTMS)-United Kingdom-Pharmaceutical Product Development, LLC