The report analyzes the market of devices by 6 segments such as cardiac rhythm management devices, electrocardiogram, holter monitors, event monitors, implantable loop recorder, and cardiac output monitoring devices. All of these segments experienced a positive growth till 2012, owing to the increased awareness for procedures and sophisticated diagnostic techniques. Cardiac rhythm management devices segments have been contributing nearly 50%, in terms of value, towards the European market.
Europe has the second largest share after North America. The economic crisis in Europe had slowed down the growth. The increase in healthcare costs, and uneven reimbursement policies across different countries in Europe are expected to be the major restraining factors for the growth of the market.
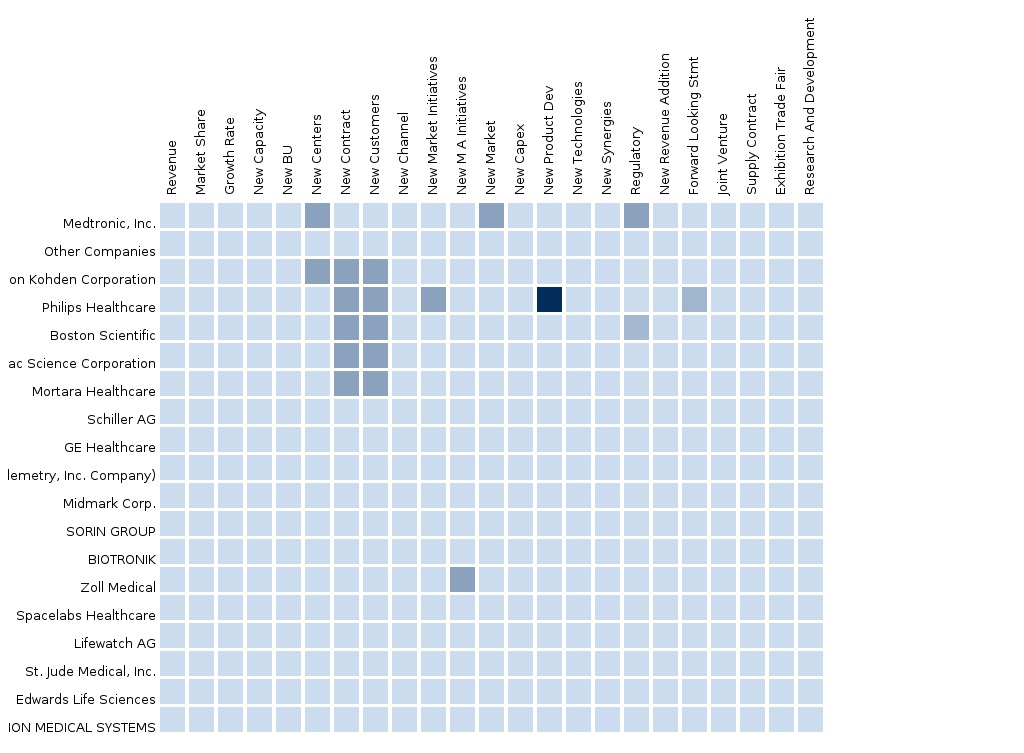
The report also provides an extensive competitive landscaping of the leading companies operating in this market. The main companies operating in the cardiac monitoring devices market and extensively covered in this report are Medtronic, St. Jude Medical, Boston Scientific, Philips Healthcare, GE Healthcare, Nihon Kohden, Cardiac Science, CardioNet, LifeWatch, Spacelabs, Mortara Instrument, Welch Allyn, and Schiller AG. The segment and country-specific company shares, news & deals, M&A, segment-specific pipeline products, product approvals, and product recalls of the major companies have been detailed.
Customization Options:
Along with market data, customize the MMM assessments to meet your company’s specific needs. Customize to get a comprehensive summary of the industry standards and a deep dive analysis of the following parameters:
Product Analysis:
- Usage pattern (in-depth trend analysis) of products (segment-wise)
- Product matrix, which gives a detailed comparison of product portfolio of each company, mapped at country and sub-segment levels
- Adoption rate analysis of the products (segment-wise and country-wise)
Epidemiology Data:
- Country-specific prevalence of CVD
- Country-specific patient pool of CVD
- Disease progression (pattern analysis)
Procedure Volume Data:
- Number of CV procedures performed in each country
- End-users
Surgeons/Physicians Perception Matrix:
- Fast turn-around analysis of surgeons’ responses to market events and trends
- Pattern analysis of usage of devices by physicians
- Surgeons’ opinions about products from different companies
- Surgeons’ qualitative inputs on epidemiology data
Brand/Product Perception Matrix:
- A comprehensive study of customers perception and behavior through our inbuilt social connect tool checking the virality and tonality of blogs
- An analysis of overall brand usage and familiarity and brand advocacy distribution (Detractor/Neutral/Familiar)
Alternative Products-Impact analysis:
- MMM’s Healthcare Decision Making Quadrant It is an innovative and useful quadrant for vendors who wish to analyze the potential growth markets based on parameters such as patient dynamics (patient pool, epidemiology of disease, preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and orthopedic clinics, reimbursement scenario, diagnosis rate, treatment rate and healthcare expenditure)
1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Cardiac Monitoring Devices-Europe, By Segments
4.1 Split By Geography
4.1 Cardiac Monitoring Devices-Spain by Markets
4.1 Cardiac Monitoring Devices-United Kingdom by Markets
4.1 Cardiac Monitoring Devices-France by Markets
4.1 Cardiac Monitoring Devices-Germany by Markets
4.1 Cardiac Monitoring Devices-Italy by Markets
4.1 Cardiac Monitoring Devices-Rest of Europe by Markets
4.2 Electrocardiogram-Europe
4.2.1 Electrocardiogram-Europe, By Geographies
4.2.1.1 Electrocardiogram-Spain
4.2.1.2 Electrocardiogram-United Kingdom
4.2.1.3 Electrocardiogram-France
4.2.1.4 Electrocardiogram-Germany
4.2.1.5 Electrocardiogram-Italy
4.2.1.6 Electrocardiogram-Rest of Europe
4.2.2 Electrocardiogram-Europe, By Segments
4.2.2.1 Resting ECG-Europe
4.2.2.2 Stress ECG-Europe
4.3 Holter Monitors-Europe
4.3.1 Holter Monitors-Europe, By Geographies
4.3.1.1 Holter Monitors-Spain
4.3.1.2 Holter Monitors-United Kingdom
4.3.1.3 Holter Monitors-France
4.3.1.4 Holter Monitors-Germany
4.3.1.5 Holter Monitors-Italy
4.3.1.6 Holter Monitors-Rest of Europe
4.3.2 Holter Monitors-Europe, By Segments
4.3.2.1 Wired Holter Monitors-Europe
4.3.2.2 Wireless Holter Monitors-Europe
4.4 Event Monitors-Europe
4.4.1 Event Monitors-Europe, By Geographies
4.4.1.1 Event Monitors-Spain
4.4.1.2 Event Monitors-United Kingdom
4.4.1.3 Event Monitors-France
4.4.1.4 Event Monitors-Germany
4.4.1.5 Event Monitors-Italy
4.4.1.6 Event Monitors-Rest of Europe
4.4.2 Event Monitors-Europe, By Services
4.4.2.1 Event Monitoring-Europe
4.4.3 Event Monitors-Europe, By Segments
4.4.3.1 Post-symptom-Europe
4.4.3.2 Pre-symptom-Europe
4.4.4 Event Monitors-Europe, By Technologies
4.4.4.1 Auto-detect Event Monitor-Europe
4.4.4.2 Manual Event Monitor-Europe
4.5 Implantable Loop Recorder-Europe
4.5.1 Implantable Loop Recorder-Europe, By Geographies
4.5.1.1 Implantable Loop Recorder-Spain
4.5.1.2 Implantable Loop Recorder-United Kingdom
4.5.1.3 Implantable Loop Recorder-France
4.5.1.4 Implantable Loop Recorder-Germany
4.5.1.5 Implantable Loop Recorder-Italy
4.5.1.6 Implantable Loop Recorder-Rest of Europe
4.6 Cardiac Output Monitoring Devices-Europe
4.6.1 Cardiac Output Monitoring Devices-Europe, By Geographies
4.6.1.1 Cardiac Output Monitoring Devices-Spain
4.6.1.2 Cardiac Output Monitoring Devices-United Kingdom
4.6.1.3 Cardiac Output Monitoring Devices-France
4.6.1.4 Cardiac Output Monitoring Devices-Germany
4.6.1.5 Cardiac Output Monitoring Devices-Italy
4.6.1.6 Cardiac Output Monitoring Devices-Rest of Europe
4.6.2 Cardiac Output Monitoring Devices-Europe, By Products
4.6.2.1 Minimally Invasive Cardiac Output Monitoring Device-Europe
4.6.2.2 Non-invasive Cardiac Output Monitoring Device-Europe
5 Cardiac Monitoring Devices-Europe, By Geographies
5.1 Cardiac Monitoring Devices-Spain
5.1.1 Cardiac Monitoring Devices-Spain, By Segments
5.1.1.1 Electrocardiogram-Spain
5.1.1.2 Holter Monitors-Spain
5.1.1.3 Event Monitors-Spain
5.1.1.4 Implantable Loop Recorder-Spain
5.1.1.5 Cardiac Output Monitoring Devices-Spain
5.2 Cardiac Monitoring Devices-United Kingdom
5.2.1 Cardiac Monitoring Devices-United Kingdom, By Segments
5.2.1.1 Electrocardiogram-United Kingdom
5.2.1.2 Holter Monitors-United Kingdom
5.2.1.3 Event Monitors-United Kingdom
5.2.1.4 Implantable Loop Recorder-United Kingdom
5.2.1.5 Cardiac Output Monitoring Devices-United Kingdom
5.3 Cardiac Monitoring Devices-France
5.3.1 Cardiac Monitoring Devices-France, By Segments
5.3.1.1 Electrocardiogram-France
5.3.1.2 Holter Monitors-France
5.3.1.3 Event Monitors-France
5.3.1.4 Implantable Loop Recorder-France
5.3.1.5 Cardiac Output Monitoring Devices-France
5.4 Cardiac Monitoring Devices-Germany
5.4.1 Cardiac Monitoring Devices-Germany, By Segments
5.4.1.1 Electrocardiogram-Germany
5.4.1.2 Holter Monitors-Germany
5.4.1.3 Event Monitors-Germany
5.4.1.4 Implantable Loop Recorder-Germany
5.4.1.5 Cardiac Output Monitoring Devices-Germany
5.5 Cardiac Monitoring Devices-Italy
5.5.1 Cardiac Monitoring Devices-Italy, By Segments
5.5.1.1 Electrocardiogram-Italy
5.5.1.2 Holter Monitors-Italy
5.5.1.3 Event Monitors-Italy
5.5.1.4 Implantable Loop Recorder-Italy
5.5.1.5 Cardiac Output Monitoring Devices-Italy
5.6 Cardiac Monitoring Devices-Rest of Europe
5.6.1 Cardiac Monitoring Devices-Rest of Europe, By Segments
5.6.1.1 Electrocardiogram-Rest of Europe
5.6.1.2 Holter Monitors-Rest of Europe
5.6.1.3 Event Monitors-Rest of Europe
5.6.1.4 Implantable Loop Recorder-Rest of Europe
5.6.1.5 Cardiac Output Monitoring Devices-Rest of Europe
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement













