About 25 in every 1000 people opt for an orthopedic surgery due to osteoarthritis. This high incidence, coupled with the aging population and increasing awareness among people drives the growth of the global orthopedic devices market.
The Asian arthroscopic devices market is mainly driven by the aging population and an increasing prevalence of osteoarthritis and the number of orthopedic surgeries.
This report analyzes the market on the basis of six segments such as arthroscopes, arthroscopic hand instrument, fluid management devices, power saver devices, radiofrequency probe, and drill guide. All these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques.
The procedure volume growth is significantly higher in Asia due to the swift disease progression, higher prevalence, improving healthcare infrastructure, economic growth, broadening insurance coverage, expanding private healthcare sector, and increasing awareness amongst people.
Minimally invasive procedures are rapidly replacing open surgeries as a result of technological innovations. Developments in visualization and monitoring equipment for instance have facilitated greater precision in joint and spinal surgeries by providing better image quality.
MIS devices are high-precision instruments made of expensive materials such as platinum, titanium, and cobalt. The leading high costs of MIS devices forms a major factor, therefore limiting the market growth. In addition, a lack of training in some cases also hinders the growth of the market in some developing countries such as India and China.
Geographically, the Asian Arthroscopic Devices Market is segmented into China, India, Japan, and Rest of Asia. In Japan, the rising rates of the obese population have been driving the procedure growth, but the Asia-wide effort to cut healthcare costs and decrease the reimbursements, will compel patients to opt for alternative surgeries such as Arthroscopic Debridement and MIS.
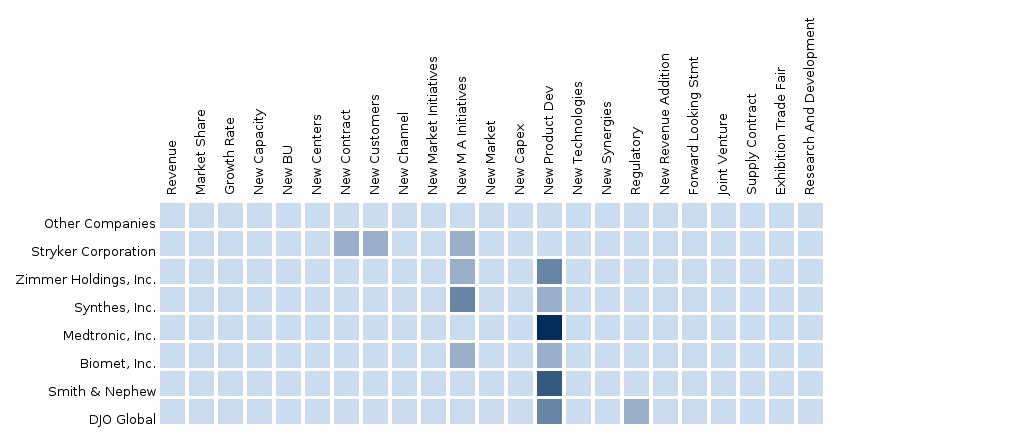
The report also provides an extensive competitive landscape of companies that operate in this market such as Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group, and Ottobock.
The segment and country-specific company shares, news & deals, M&A, segment specific pipeline products, product approvals, and product recalls of the major companies have been provided in detail.
Customization Options:
Along with the market data, you can also customize the MMM assessments that meet your company’s specific requirements. You can customize to get comprehensive industry standards and a DeepDive analysis of the following parameters:
Product Analysis
- Usage pattern (in-depth trend analysis) of products (segment-wise)
- Product matrix that provides a detailed comparison of the product portfolios of each company that has been mapped at the country and sub-segment level
- End-user adoption rate analysis of the products (segment wise and country wise)
- A comprehensive coverage of product approvals, pipeline products, and product recalls
Epidemiology Data
- Country specific prevalence of knee, hand, hip, spine osteoarthritis (OA), rheumatoid arthritis (RA), and diabetes.
- Country specific patient pool of knee, hand, hip, spine OA, and RA
- Disease progression (pattern analysis)
Procedure Volume Data
- Bilateral and unilateral hip and knee procedures performed annually in each country that have been tracked till the sub-segment level
- Number of foot & ankle, wrist, shoulder, elbow, digit, and disc replacement procedures performed in each country
Surgeons/Physicians Forum
- Fast turn-around analysis of surgeons’ response to the market events and trends
- Pattern analysis of the usage of bone cements (antibiotic and non-antibiotic) by physicians
- Surgeons’ opinion about products from different companies
- Surgeons’ qualitative inputs on epidemiology data
Perception Matrix
- A comprehensive study of the customers’ perception and behavior through our inbuilt social connect tool that checks the virality and tonality of blogs
- Analysis of the overall brand usage, familiarity, and brand advocacy distribution (Detractor/Neutral/Familiar)
Alternative Products: Impact analysis
MMM’s Healthcare Decision Making Quadrant is an innovative and useful quadrant for vendors who wish to analyze the potential growth markets based on parameters such as patient dynamics (patient pool, epidemiology of disease, preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and orthopedic clinics, reimbursement scenario, diagnosis rate, treatment rate, and healthcare expenditure).
1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Arthroscopic Devices-Asia, By Segments
4.1 Split By Geography
4.1 Arthroscopic Devices-Asia - Japan by Markets
4.1 Arthroscopic Devices-China by Markets
4.1 Arthroscopic Devices-India by Markets
4.2 Arthroscopes-Asia
4.2.1 Arthroscopes-Asia, By Endusers
4.2.1.1 Arthroscopes-Asia-Hospitals
4.2.1.2 Arthroscopes-Asia-Point of Care Testing
4.2.1.3 Arthroscopes-Asia-Other Endusers
4.2.2 Arthroscopes-Asia, By Geographies
4.2.2.1 Arthroscopes-Asia - Japan
4.2.2.2 Arthroscopes-China
4.2.2.3 Arthroscopes-India
4.3 Arthroscopic Hand Instruments-Asia
4.3.1 Arthroscopic Hand Instruments-Asia, By Endusers
4.3.1.1 Arthroscopic Hand Instruments-Asia-Hospitals
4.3.1.2 Arthroscopic Hand Instruments-Asia-Point of Care Testing
4.3.1.3 Arthroscopic Hand Instruments-Asia-Other Endusers
4.3.2 Arthroscopic Hand Instruments-Asia, By Geographies
4.3.2.1 Arthroscopic Hand Instruments-Asia - Japan
4.3.2.2 Arthroscopic Hand Instruments-China
4.3.2.3 Arthroscopic Hand Instruments-India
4.4 Fluid Management Devices-Asia
4.4.1 Fluid Management Devices-Asia, By Endusers
4.4.1.1 Fluid Management Devices-Asia-Hospitals
4.4.1.2 Fluid Management Devices-Asia-Point of Care Testing
4.4.1.3 Fluid Management Devices-Asia-Other Endusers
4.4.2 Fluid Management Devices-Asia, By Geographies
4.4.2.1 Fluid Management Devices-Asia - Japan
4.4.2.2 Fluid Management Devices-China
4.4.2.3 Fluid Management Devices-India
4.5 Power Saver Systems-Asia
4.5.1 Power Saver Systems-Asia, By Endusers
4.5.1.1 Power Saver Systems-Asia-Hospitals
4.5.1.2 Power Saver Systems-Asia-Point of Care Testing
4.5.1.3 Power Saver Systems-Asia-Other Endusers
4.5.2 Power Saver Systems-Asia, By Geographies
4.5.2.1 Power Saver Systems-Asia - Japan
4.5.2.2 Power Saver Systems-China
4.5.2.3 Power Saver Systems-India
4.6 Radiofrequency Probe-Asia
4.6.1 Radiofrequency Probe-Asia, By Endusers
4.6.1.1 Radiofrequency Probe-Asia-Hospitals
4.6.1.2 Radiofrequency Probe-Asia-Point of Care Testing
4.6.1.3 Radiofrequency Probe-Asia-Other Endusers
4.6.2 Radiofrequency Probe-Asia, By Geographies
4.6.2.1 Radiofrequency Probe-Asia - Japan
4.6.2.2 Radiofrequency Probe-China
4.6.2.3 Radiofrequency Probe-India
4.7 Drill Guide-Asia
4.7.1 Drill Guide-Asia, By Endusers
4.7.1.1 Drill Guide-Asia-Hospitals
4.7.1.2 Drill Guide-Asia-Point of Care Testing
4.7.1.3 Drill Guide-Asia-Other Endusers
4.7.2 Drill Guide-Asia, By Geographies
4.7.2.1 Drill Guide-Asia - Japan
4.7.2.2 Drill Guide-China
4.7.2.3 Drill Guide-India
5 Arthroscopic Devices-Asia, By Endusers
5.1 Split By Geography
5.2 Arthroscopic Devices-Asia - Japan by Endusers
5.1 Arthroscopic Devices-China by Endusers
5.1 Arthroscopic Devices-India by Endusers
5.2 Arthroscopic Devices-Asia-Hospitals
5.2.1 Arthroscopic Devices-Asia-Hospitals, By Geographies
5.2.1.1 Arthroscopic Devices-Asia - Japan-Hospitals
5.2.1.2 Arthroscopic Devices-China-Hospitals
5.2.1.3 Arthroscopic Devices-India-Hospitals
5.2.2 Arthroscopic Devices-Asia-Hospitals, By Segments
5.2.2.1 Arthroscopes-Asia-Hospitals
5.2.2.2 Arthroscopic Hand Instruments-Asia-Hospitals
5.2.2.3 Fluid Management Devices-Asia-Hospitals
5.2.2.4 Power Saver Systems-Asia-Hospitals
5.2.2.5 Radiofrequency Probe-Asia-Hospitals
5.2.2.6 Drill Guide-Asia-Hospitals
5.3 Arthroscopic Devices-Asia-Point of Care Testing
5.3.1 Arthroscopic Devices-Asia-Point of Care Testing, By Geographies
5.3.1.1 Arthroscopic Devices-Asia - Japan-Point of Care Testing
5.3.1.2 Arthroscopic Devices-China-Point of Care Testing
5.3.1.3 Arthroscopic Devices-India-Point of Care Testing
5.3.2 Arthroscopic Devices-Asia-Point of Care Testing, By Segments
5.3.2.1 Arthroscopes-Asia-Point of Care Testing
5.3.2.2 Arthroscopic Hand Instruments-Asia-Point of Care Testing
5.3.2.3 Fluid Management Devices-Asia-Point of Care Testing
5.3.2.4 Power Saver Systems-Asia-Point of Care Testing
5.3.2.5 Radiofrequency Probe-Asia-Point of Care Testing
5.3.2.6 Drill Guide-Asia-Point of Care Testing
5.4 Arthroscopic Devices-Asia-Other Endusers
5.4.1 Arthroscopic Devices-Asia-Other Endusers, By Geographies
5.4.1.1 Arthroscopic Devices-Asia - Japan-Other Endusers
5.4.1.2 Arthroscopic Devices-China-Other Endusers
5.4.1.3 Arthroscopic Devices-India-Other Endusers
5.4.2 Arthroscopic Devices-Asia-Other Endusers, By Segments
5.4.2.1 Arthroscopes-Asia-Other Endusers
5.4.2.2 Arthroscopic Hand Instruments-Asia-Other Endusers
5.4.2.3 Fluid Management Devices-Asia-Other Endusers
5.4.2.4 Power Saver Systems-Asia-Other Endusers
5.4.2.5 Radiofrequency Probe-Asia-Other Endusers
5.4.2.6 Drill Guide-Asia-Other Endusers
6 Arthroscopic Devices-Asia, By Geographies
6.1 Arthroscopic Devices-Asia - Japan
6.1.1 Arthroscopic Devices-Asia - Japan, By Endusers
6.1.1.1 Arthroscopic Devices-Asia - Japan-Hospitals
6.1.1.2 Arthroscopic Devices-Asia - Japan-Point of Care Testing
6.1.1.3 Arthroscopic Devices-Asia - Japan-Other Endusers
6.1.2 Arthroscopic Devices-Asia - Japan, By Segments
6.1.2.1 Arthroscopes-Asia - Japan
6.1.2.2 Arthroscopic Hand Instruments-Asia - Japan
6.1.2.3 Fluid Management Devices-Asia - Japan
6.1.2.4 Power Saver Systems-Asia - Japan
6.1.2.5 Radiofrequency Probe-Asia - Japan
6.1.2.6 Drill Guide-Asia - Japan
6.2 Arthroscopic Devices-China
6.2.1 Arthroscopic Devices-China, By Endusers
6.2.1.1 Arthroscopic Devices-China-Hospitals
6.2.1.2 Arthroscopic Devices-China-Point of Care Testing
6.2.1.3 Arthroscopic Devices-China-Other Endusers
6.2.2 Arthroscopic Devices-China, By Segments
6.2.2.1 Arthroscopes-China
6.2.2.2 Arthroscopic Hand Instruments-China
6.2.2.3 Fluid Management Devices-China
6.2.2.4 Power Saver Systems-China
6.2.2.5 Radiofrequency Probe-China
6.2.2.6 Drill Guide-China
6.3 Arthroscopic Devices-India
6.3.1 Arthroscopic Devices-India, By Endusers
6.3.1.1 Arthroscopic Devices-India-Hospitals
6.3.1.2 Arthroscopic Devices-India-Point of Care Testing
6.3.1.3 Arthroscopic Devices-India-Other Endusers
6.3.2 Arthroscopic Devices-India, By Segments
6.3.2.1 Arthroscopes-India
6.3.2.2 Arthroscopic Hand Instruments-India
6.3.2.3 Fluid Management Devices-India
6.3.2.4 Power Saver Systems-India
6.3.2.5 Radiofrequency Probe-India
6.3.2.6 Drill Guide-India
7 Arthroscopic Devices-Asia, By Companies
7.1 Competitive landscape
7.2 Split By Geography
7.3 Arthroscopic Devices-Asia - Japan by Companies
7.1 Arthroscopic Devices-China by Companies
7.1 Arthroscopic Devices-India by Companies
7.3 Arthroscopic Devices-Asia-Other Companies
7.4 Arthroscopic Devices-Asia-Stryker Corporation
7.5 Arthroscopic Devices-Asia-Zimmer Holdings, Inc.
7.6 Arthroscopic Devices-Asia-Synthes, Inc.
7.7 Arthroscopic Devices-Asia-Medtronic, Inc.
7.8 Arthroscopic Devices-Asia-Biomet, Inc.
7.9 Arthroscopic Devices-Asia-Smith & Nephew
7.10 Arthroscopic Devices-Asia-DJO Global
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit https://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
Asia Orthopedics Device Market The report “Asian Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. The main companies operating in Asian Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
North America Orthopedic Devices The report “North American Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques. Both the markets have been witnessing the maximum growth because of increase in patient pool and procedure numbers of hip and knee osteoarthritis and rheumatoid arthritis. The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in North American Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. ... |
Upcoming |
 |
Europe Orthopedics Device Market The report “European Orthopedic Devices Market forecast, 2012-2018 “analyzes the market of devices by 8 segments such as Orthopedic Braces & Support Systems, Joint Reconstruction Devices, Trauma Fixation Devices, Arthroscopic Devices, Spine Surgery Devices, Orthobiologics and Orthopedic Accessories. All of these segments experienced a positive growth till 2012 with an increased awareness for procedures and sophisticated diagnostic techniques.The report also provides an extensive competitive landscaping of companies operating in this market. The main companies operating in European Orthopedic Devices market and extensively covered in this report are Stryker, Zimmer, DePuy Synthes, Medtronic, Smith and Nephew, Exactech Inc., Tornier, Biomet Inc., Wright Medical Group and Ottobock. |
Upcoming |












